Figure 2.
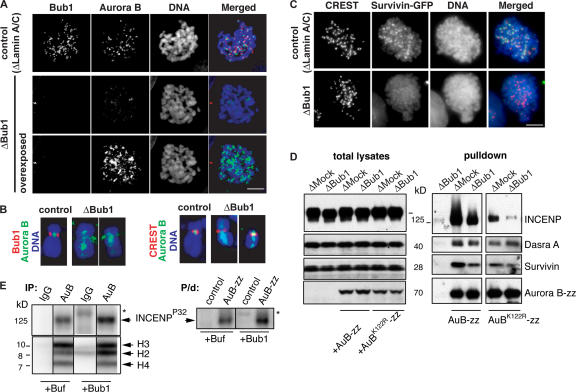
Bub1 is required for the inner centromeric localization of CPC in somatic cells. HeLa cells were transfected with Lamin A/C or Bub1 siRNA duplexes for 24 h and treated with nocodazole for 1 h before fixation. (A) Cells were stained to detect Bub1 (red) and Aurora B (green). (B) Individual chromosomes are shown in higher magnification to allow comparison of the Aurora B staining pattern to that of Bub1 or to the centromere marker (CREST). DNA was visualized as in Fig. 1. Note that the acquired signal of Aurora B staining in Bub1-depleted cells was overexposed. (C) HeLa cells stably expressing GFP-Survivin were treated as in A, and the position of GFP signal pattern was compared with that of CREST. (D) Bub1 is required for stability of CPC. Recombinant wtAurora B-zz or Aurora BK122R–zz were added to control or Bub1-depleted CSF extracts at a concentration approximately equal to that of endogenous Aurora B, and CPC complexes that formed on Aurora B-zz were purified by IgG–Sepharose beads. Total extracts (left) and eluates (right) were probed for the presence of CPC components. (E) Bub1 phosphorylates INCENP. CPC was either precipitated from Bub1-depleted egg extracts using antibodies against Aurora B (IP; left) or was purified on IgG–Sepharose from extracts, supplemented with Aurora B-zz (P/d, right). Mock-treated beads and beads containing precipitates were incubated with baculovirus-expressed 6His-xBub1 (50 μM) or buffer in the presence of γ[32P]ATP. Phosphate incorporation was detected using a PhosphorImager. The asterisks show the position of 6His-xBub1 (top). Immunoprecipitates (as in Fig. 1) were also incubated with core histones as exogenous substrates in the presence or absence of 6His-xBub1 to monitor Aurora B activity (bottom).