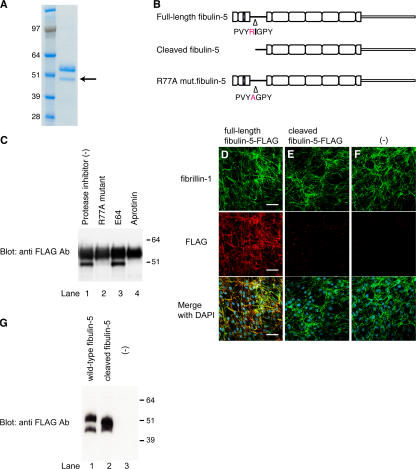
Figure 5.
Fibulin-5 is cleaved after the arginine at position 77 by serine protease and loses the microfibril-associating activity. (A) 293T cells were stably transfected with an expression vector encoding C-terminal-FLAG– and 6× histidine-tagged fibulin-5 cDNA. Recombinant fibulin-5 protein was purified by chelating chromatography from the culture media of these cells, subjected to SDS-PAGE, and stained with Coomassie blue. The lower band (arrow) was subjected to N-terminal sequencing. (B) The N-terminal sequencing of the lower band identified the specific cleavage site of fibulin-5 at the arginine at position 77. (C) 293T cells were transiently transfected with the expression vector encoding C-terminal-FLAG– and 6× histidine-tagged fibulin-5 or R77A mutant fibulin-5, which had a mutation from arginine to alanine at position 77. Transfected cells were cultured for 2 d with or without a cysteine protease inhibitor, E64, or a serine protease inhibitor, aprotinine, added to the culture media. Culture media were then harvested, concentrated by chelating chromatography, and subjected to Western blotting with anti-FLAG antibody. (D–G) Human skin fibroblasts were cultured for 4 d in serum-free medium in the presence of recombinant fibulin-5 (D) or recombinant cleaved fibulin-5 (E) proteins at a concentration of 4 μg/ml in the medium, or without recombinant protein (F). The cells were then double-stained with anti–fibrillin-1 polyclonal antibody (top) and with anti-FLAG monoclonal antibody (middle). Bottom images were produced by superimposition of the top and middle images, together with DAPI nuclear staining. Bars, 60 μm. At the same time, the conditioned medium was immunoblotted with anti-FLAG antibody to confirm that neither fibulin-5 nor cleaved fibulin-5 was degraded during the culture period (G).