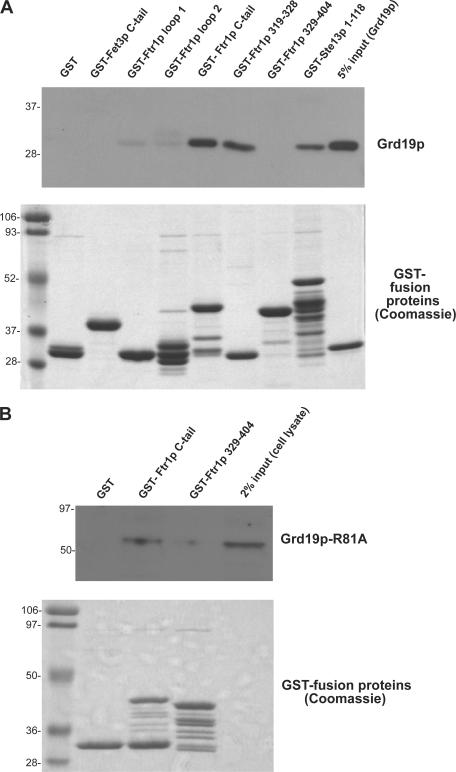
Figure 5.
Grd19p binds directly to the Ftr1p recycling signal. (A) The following GST-fusion proteins were expressed and purified from bacteria and immobilized on glutathione–Sepharose beads: GST-Fet3p cytoplasmic tail (residues 581–636), GST-Ftr1p cytoplasmic loop 1 (residues 30–48), GST-Ftr1p cytoplasmic loop 2 (residues 109–153), GST-Ftr1p cytoplasmic tail (residues 315–404), GST-Ftr1p(319–328), which is the putative recycling signal, and GST-Ftr1p(329–404), which contains the entire C-terminal domain except for the recycling signal. Purified fusion proteins were incubated with purified recombinant His6-Grd19p, which contains a T7 epitope tag on the N terminus. The beads were washed, and bound proteins were eluted in SDS sample buffer. After SDS-PAGE, Grd19p was detected using an anti-T7 epitope antibody (top) and the GST fusion proteins were visualized by Coomassie blue staining (bottom). (B) Yeast strains expressing Grd19p-R81A-myc and the indicated GST fusion proteins were grown in synthetic media containing 4% galactose. Cells were harvested, converted to spheroplasts, and the bifunctional chemical cross-linking reagent DSP was added to a final concentration of 4 mM. The cells were then lysed, and the GST fusion proteins were captured on glutathione–Sepharose beads. The cross-links were disrupted by reduction in SDS sample buffer, and the samples were probed by immunoblotting with anti-myc antibodies (top). GST fusion proteins were visualized by Coomassie blue staining (bottom).