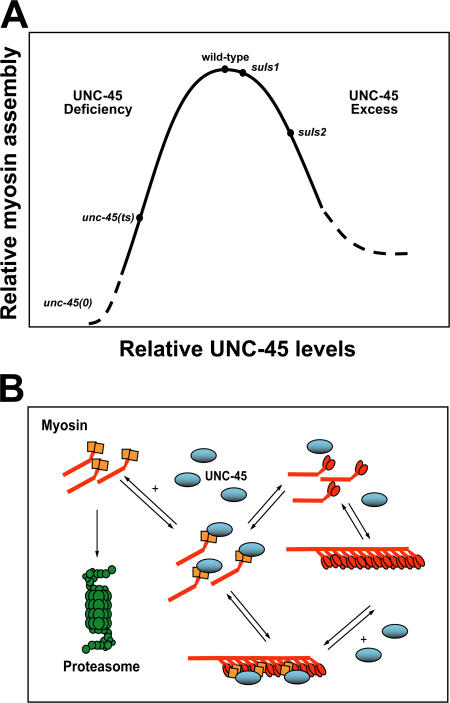
Figure 4.
Mechanism of UNC-45 chaperone–mediated proteasomal degradation of myosin. (A) Myosin assembly as an idealized function of UNC-45 chaperone levels. The two are linked by control of myosin accumulation through its degradation. ts, temperature sensitive. (B) Proposed mechanism of UNC-45 chaperone–mediated myosin assembly, disassembly, and consequent degradation. All reactions represented by opposing arrows are reversible; myosin degradation represented by a single arrow is irreversible. By mass action, chaperone deficiency leads to a buildup of free, nonnative myosin, a substrate for the proteasome. Similarly, excess chaperone leads to a buildup of its complexes with myosin molecules and filaments. These complexes lead to increased levels of free, nonnative myosin and its proteasomal degradation.