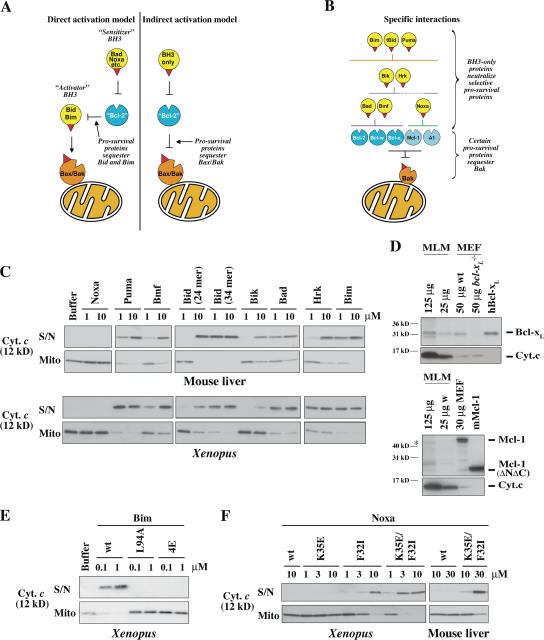
Figure 1.
Specificity of BH3 peptide–induced cytochrome c release. (A) Prosurvival proteins are proposed to block mitochondrial permeability by sequestering BH3-only proteins (direct activation model) or primarily Bax/Bak proteins (indirect activation model). (B) Previously identified specific interactions between members of each Bcl-2 subfamily (Fig. S1 B, available at http://www.jcb.org/cgi/content/full/jcb.200606065/DC1; Chen et al., 2005). (C) Selective permeabilization by BH3 peptides. Mitochondria isolated from mouse liver or from Xenopus eggs were incubated for 2 h at 37 (mouse liver) or 22°C (Xenopus) with peptides derived from the BH3 domains of BH3-only proteins. Equivalent volumes of supernatant (S/N) and mitochondrial pellet (Mito) were then analyzed for cytochrome c by Western blotting. The sequence of each peptide is from the human protein sequence (Fig. S1 A) except for Bmf (from mouse) and Bad (human in top panel and mouse in bottom panel). BH3 peptides derived from mouse and human Bad were equivalent in permeabilizing and binding activity (not depicted) and were used interchangeably. (D) Bcl-xl and traces of Mcl-1 are present in MLM. Bcl-xl and Mcl-1 levels in MLM and mouse embryonic fibroblasts (MEFs) were compared by Western blotting. 0.7 pmol of recombinant Bcl-xL and 0.25 pmol Mcl-1 were included where indicated. Positions of molecular weight markers are indicated. The asterisk denotes a band in MLM that aligns with the bottom band of the Mcl-1 doublet in the mouse embryonic fibroblast. Reprobing the blots for cytochrome c (bottom) provides a comparison of the mitochondrial content of each sample. (E) Mutant Bim peptides lose permeabilizing activity. Mouse BimBH3 peptides with mutations at one (L94A) or four (4E; I89E, L94E, I97E, and F101E) conserved residues were compared with wild-type (wt) peptide for the ability to permeabilize XEM as in C (residue number refers to the mBimL protein). Equivalent results were found with MLM (not depicted). (F) Mutated Noxa peptides with enhanced binding to Bcl-xL gain permeabilizing activity. NoxaBH3 peptides containing substitutions of charge (K35E), hydrophobicity (F32I), or both (K35E and F32I; Fig. S1; Chen et al., 2005) were compared with wild-type peptide for the ability to permeabilize mitochondria as in C.