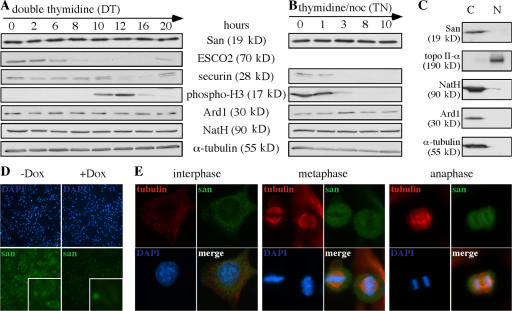
Figure 5.
Cell cycle regulation and cellular localization of San. (A and B) The protein level of San is unchanged throughout the cell cycle. HeLa cells were synchronized at the G1–S transition by the double thymidine arrest protocol (A) and at c-metaphase by the thymidine–nocodazole arrest protocol (B) and released into the cell cycle. ESCO2, phospho-histone H3, and securin were blotted as the markers for different stages of the cell cycle. Specifically, the degradation of ESCO2 (Hou and Zou, 2005) commences at late S phase (6 h after release from DT); histone H3 becomes phosphorylated in mitosis (10–12 h after release from DT and 0–3 h after release from TN); and securin is degraded in anaphase and early G1 (12–16 h after release from DT and 3–10 h after release from TN). (C) San is detected in the cytoplasmic fraction. HeLa cells were fractionated into the cytoplasm (C) and the nuclear (N) fractions. Topoisomerase II and α-tubulin are markers for the nuclear and cytoplasm fractions, respectively. As expected, NatA and Ard1 were also detected in the cytoplasm (Arnesen et al., 2005). (D) The antibody to San detected San and its cytoplasmic location (insets) in the uninduced (−Dox), but not in the induced (+Dox), San-shRNA cells. San-shRNA cells were incubated under induced and uninduced conditions for 5 d before being analyzed. (E) The localization of San, as determined by immunofluorescent microscopy. The localization of San in HeLa cells was analyzed in the cells at interphase, metaphase, and anaphase.