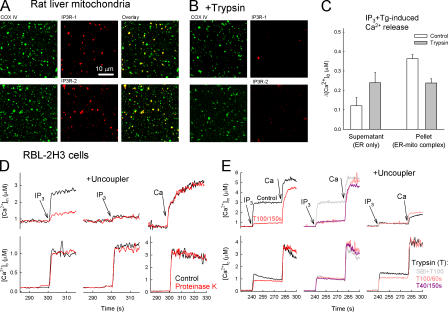
Figure 2.
Limited proteolysis loosens the structural and functional association of the IP3R with mitochondria. (A and B) Confocal images showing the distribution of IP3Rs (red) and the cytochrome c oxidase (green) and their colocalization (overlay, yellow) in a rat-liver mitochondrial fraction attached to coverslips. (B) Trypsin (40 μg/ml; 150 s) was added before attachment to the coverslips (n = 3). (C) ER Ca2+ storage in naive and trypsin-pretreated rat-liver mitochondrial fraction. In suspensions of the particles, the capacity of the ER Ca2+ store was determined as the sum of the extravesicular [Ca2+] ([Ca2+]o) increases caused by sequentially added IP3 and Tg in the 10,000-g supernatants (ER-only fraction) and pellets (ER–mitochondria complex) after trypsinization (40 μg/ml; 150 s) in the presence (control) and absence of SBI (mean ± SEM; n = 10). (D and E) Effect of proteinase K and trypsin on the IP3-induced [Ca2+]c and [Ca2+]m increase in suspensions of permeabilized RBL-2H3 cells. (D) Control (black) and proteinase K–pretreated cells (red) in the absence (left) or presence (middle) of uncoupler (2 μM carbonyl cyanide p-trifluoromethoxyphenylhydrazone + 5 μg/ml oligomycin). (right) [Ca2+]m rise evoked by a 10 μM CaCl2 pulse (Ca; bulk [Ca2+]c increase, ∼3 μM). To prevent the uptake of added Ca2+ by the ER, 2 μM Tg was added 5 s before stimulus. (E) 100 μg/ml trypsin for 150 s (left, red) or 60 s (middle, pink). 40 μg/ml trypsin for 150 s (middle) in the absence (purple) or presence (gray) of 250 μg/ml SBI. (right) Effect of trypsin in the presence of uncoupler.