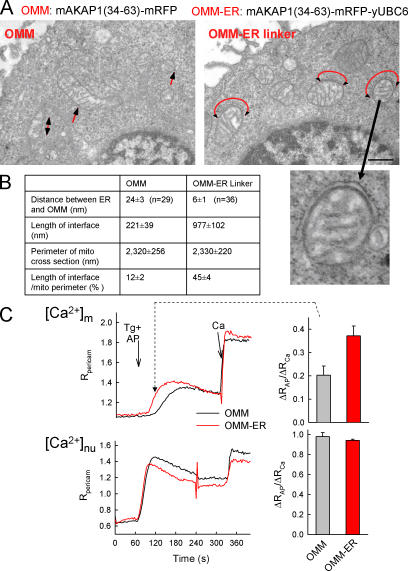
Figure 3.
Enhancement of the ER–mitochondria association and Ca2+ coupling by a synthetic linker protein. (A) Electron micrographs of RBL-2H3 cells expressing the mAKAP1(34–63)-mRFP-yUBC6 or mAKAP1(34–63)-mRFP with red arrows showing the ER–mitochondria contacts. (B) Dimensions of the ER–mitochondria interface in each condition. (C) [Ca2+]m and nuclear [Ca2+] ([Ca2+]nu) responses to submaximal doses of adenophostin (AP) recorded using pericam in cells transfected with OMM-mRFP (black) or OMM–ER linker-mRFP (red). Adenophostin evokes gradual Ca2+ liberation through IP3Rs and a [Ca2+]m increase characterized with a gradual slow phase when the sarcoplasmic/endoplasmic reticulum calcium ATPase pumps are blocked (Csordas and Hajnoczky, 2001). As a reference, a 20-μM CaCl2 pulse (Ca) was applied. (right) [Ca2+]c and [Ca2+]m increases 60 s after adenophostin stimulation. Data are normalized to the response evoked by Ca (P > 0.01; n = 15–16). Error bars indicate SEM.