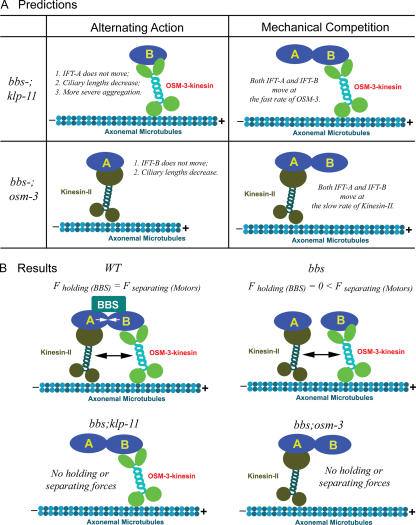
Figure 3.
Model showing BBS proteins antagonizing mechanical competition between kinesin-II and OSM-3 to maintain IFT particle integrity. (A) Demonstrates distinct phenotypes predicted by the two models in bbs-;motor double mutants. The alternating action model predicts that in bbs-;klp-11 double mutants, IFT-A cannot be moved by either kinesin-II or OSM-3–kinesin and will not enter cilia, so IFT-A will form aggregates in the endings of truncated cilia, mimicking the phenotype of IFT-A mutants. On the other hand, in bbs-;osm-3 double mutants, IFT-B cannot be moved by kinesin-II or OSM-3, and ciliary length will decrease. In contrast, the mechanical competition model predicts that in either bbs-;klp-11 or bbs-;osm-3 double mutants, there will be no mechanical competition between the two motors or no drag exerted through IFT particles, so even in the absence of BBS proteins, IFT particles can be maintained in a single complex, and A and B subcomplexes will display identical transport profiles. (B) Summary of the results of transport assays that test the predictions (Fig. 4). In wild type (WT), BBS proteins maintain IFT particle integrity by antagonizing the mechanical competition between kinesin-II and OSM-3. In bbs-7/-8 single mutants, mechanical competition between kinesin-II and OSM-3 is not counterbalanced by BBS proteins, so IFT particles dissociate into subcomplexes A and B. In bbs-7/-8;kinesin-II or bbs-7/-8;osm-3 double mutants, no mechanical competition is generated, so IFT particles do not dissociate but are moved by kinesin-II or OSM-3 alone.