Figure 5.
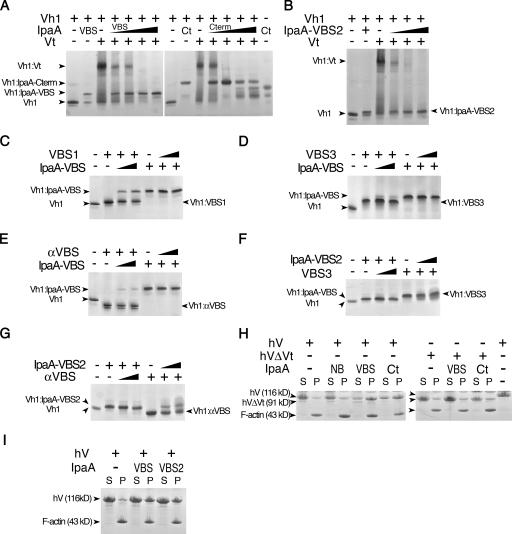
S. flexneri's IpaA-VBSs activate vinculin. (A) S. flexneri's IpaA-VBSs and its C-terminal domain (Ct) sever the Vh1–Vt interaction that clamps vinculin in its inactive state (Borgon et al., 2004; Izard et al., 2004). Lane 1 in both the left and right native gels show Vh1 alone, and lane 2 shows the Vh1–IpaA-VBS complex (left) or the Vh1–IpaA-Cterm complex (right). The + denotes the addition of the indicated IpaA domain to preformed Vh1–Vt complexes. Competing IpaA-VBS (left) was titrated (lanes 4–7, arrowheads) into preformed Vh1–Vt complexes at molar ratios of IpaA-VBS at ∼2-, 3.5-, 10-, and 20-fold molar ratios (Vh1–IpaA-VBS). Competing IpaA-Cterm peptide (right) was titrated (arrows) into preformed Vh1–Vt complexes at 0.9-, 1.7-, 3.4-, and 6.8-fold molar ratios (Vh1–IpaA-Cterm). As is evident, titration of even very low molar ratios of IpaA-VBS or IpaA-Cterm was sufficient to totally disrupt the Vh1–Vt complex to form novel Vh1–IpaA-VBS (left) or Vh1–IpaA-Cterm (right) complexes. Because of its basic pI, free Vt is not visible in native gels (Izard et al., 2004). (B) IpaA-VBS2 is also sufficient to disrupt the Vh1–Vt interaction. Native gel analyses of free Vh1 (lane 1), Vh1 in complex with IpaA-VBS2 (lane 2), Vh1–Vt complex (lane 3), or preexisting Vh1–Vt complexes titrated with increasing concentrations of IpaA-VBS2 (at ∼2-, 3.5-, 10-, and 20-fold molar ratios; Vh1–IpaA-VBS2). The identity of the complexes is shown. (C–G) The selective nature of the IpaA–vinculin interaction. Reciprocal VBS displacement native gel assays of Vh1 in complex with talin-VBS1 (C), talin-VBS3 (D and F), or the VBS of α-actinin (αVBS; E and G) versus when in complex with IpaA-VBS (C–E) or IpaA-VBS2 (F and G). Competing IpaA-VBS, IpaA-VBS2, talin-VBS3, talin-VBS1, or αVBS peptides were titrated (arrows) into preformed complexes at 2.2- or 10-fold molar excess (Vh1–VBS). Native gels of the indicated complexes are shown. (H and I) IpaA-VBS (H) and -VBS2 (I) promote vinculin binding to F-actin. IpaA-NB, -VBS (VBS), -VBS2 (VBS2), or -Cterm (Ct) were incubated with full-length human vinculin (hV) or human vinculin lacking the tail domain Vt (hVΔVt), which mediates binding to F-actin (Johnson and Craig, 1995). Samples were incubated with F-actin, as previously described (Bois et al., 2006), and centrifuged into pellet (containing polymerized F-actin) and supernatant fractions, and then equal volumes were analyzed by SDS-PAGE. F-actin and vinculin were visualized by staining the gels with Coomassie blue. The last lane on the right of H shows the size difference between full-length vinculin (residues 1–1,066) and the head domain of vinculin (residues 1–840).