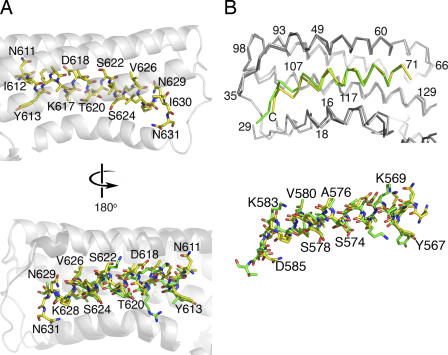
Figure 6.
The mechanism of vinculin activation by S. flexneri's IpaA protein. (A, top) The crystal structure of the Vh1–IpaA-VBS complex showing the hydrophobic face of IpaA-VBS (yellow bonds; some residues are labeled) that interacts with vinculin (transparent white helices). Vinculin is shown as a ribbon diagram in white, and the individual bonds are drawn in yellow for IpaA-VBS (oxygen atoms, red; carbon, yellow; nitrogen, blue). (bottom) Superposition of talin-VBS3 (green bonds) onto IpaA-VBS (yellow bonds) bound to vinculin (white and gray, respectively). This orientation is rotated by 180° in respect to the top image showing the hydrophilic solvent–exposed face of these VBSs. Some IpaA-VBS residues are labeled. (B) (top) The Cα trace of the Vh1 domain of vinculin bound to IpaA-VBS (yellow) superimposed onto IpaA-VBS2 (green) bound to Vh1 in gray (Vh1–IpaA-VBS2) or white (Vh1–IpaA-VBS). Some vinculin residues are labeled. (bottom) Ball-and-stick representation of the structure of IpaA-VBS (yellow) superimposed onto that of IpaA-VBS2 (green); some residues of IpaA-VBS2 are labeled. Both images are shown in the same orientation.