Abstract
Interactions between Schwann cells and axons are critical for the development and function of myelinated axons. Two recent studies (see Maurel et al. on p. 861 of this issue; Spiegel et al., 2007) report that the nectin-like (Necl) proteins Necl-1 and -4 are internodal adhesion molecules that are critical for myelination. These studies suggest that Necl proteins mediate a specific interaction between Schwann cells and axons that allows proper communication of the signals that trigger myelination.
The myelin sheath allows the fast transmission of action potentials over long distances in vertebrates. In the peripheral nervous system, glial cells termed Schwann cells tightly associate with axons and form myelin (Sherman and Brophy, 2005). Although the word glia derives from the Greek for glue, little is known about the molecules that play a role in axo–glial adhesion along the internode, which constitutes the great majority of the myelinated axon (Poliak and Peles, 2003; Salzer, 2003). Maurel et al. (2007) and Spiegel et al. (2007) now report that Necl proteins are internodal adhesion molecules that are required for myelination.
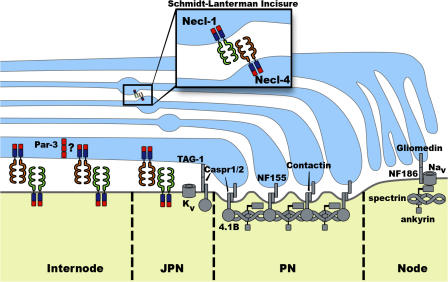
Interactions between Schwann cells and axons are critical for the development and function of myelinated axons. Axonal signals, including neuregulins, control the proliferation and migration of immature Schwann cells, which initially ensheath bundles of growing axons (Jessen and Mirsky, 2005). As development progresses, a promyelinating Schwann cell interacts more stably with a part of a single axon, around which it forms an internodal myelin segment (Jessen and Mirsky, 2005). Axonal neuregulin signals also regulate the commitment of Schwann cells to myelination and ensure that the thickness of the myelin is appropriate for a particular axon (Michailov et al., 2004; Taveggia et al., 2005). Reciprocally, signals from Schwann cells initiate the formation of the different functional domains in myelinated axons, including the node of Ranvier (Poliak and Peles, 2003; Salzer, 2003). Recent work has identified molecules that mediate axo–glial communication at the nodes, paranodes, and juxtaparanodes (summarized in Fig. 1; Poliak and Peles, 2003; Salzer, 2003), but less is known about the molecules that play a role in adhesion and communication along the length of the internode. Moreover, it has been unclear what molecules bring about changes in the association between axons and Schwann cells over time, as Schwann cells cease migration, transition from ensheathing many axons to just one, and initiate myelination.
Figure 1.
Model of interactions between myelinating Schwann cells and axons showing proteins that localize to the node, paranode, juxtaparanode, and internode. Schwann cell, blue; axon, yellow. PN, paranode; JPN, juxtaparanode. Analysis of the Necl proteins provides important new insight into molecular complexes along the internode (Maurel et al., 2007; Spiegel et al., 2007), which had not been as well defined as for the other domains. The polarity protein Par-3 colocalizes with Necl-4 in the adaxonal region of the Schwann cell (Chan et al., 2006), and it is possible that these proteins interact via PDZ and PDZ-binding motifs (red). The Necl proteins can also interact with FERM domain proteins via the FERM-binding domain (blue). Necls are also present at the Schmidt-Lanterman incisures (inset). Necl-2 is not depicted but localizes to the axon–Schwann cell interface and to the incisures. The figure was adapted from Voas et al. (2007).
Necl proteins are a recently defined family of adhesion molecules characterized by an extracellular domain comprising three Ig-like loops, a transmembrane domain, and a short cytoplasmic tail (Takai et al., 2003). Through motifs in the cytoplasmic tail, Necls can interact with a variety of proteins containing PDZ (PSD-95, DLG, Z01) or FERM (4.1, ezrin, radixin, moesin) domains (Takai et al., 2003). There are five known Necls, Necl-1–5, and they are involved in many processes, including cell motility, adhesion, and synaptogenesis. Necls participate in both homophilic and heterophilic interactions, and, in addition, Necls also bind to the related nectins, which can, in turn, mediate associations with additional adhesion proteins. Necl-1 is expressed specifically in the nervous system, whereas Necl-4 is expressed more widely in tissues including the brain, kidney, and prostate (Fukuhara et al., 2001; Kakunaga et al., 2005). In addition, Necl-4 was identified in a screen for signal sequence–containing proteins expressed in Schwann cells (Spiegel et al., 2006).
Maurel et al. (2007) and Spiegel et al. (2007) show that Necl proteins are differentially expressed in axons and Schwann cells (Fig. 1). Axons express Necl-1 and -2, whereas Schwann cells express Necl-2 and -4. Necl-4 expression is up-regulated in Schwann cells as myelination is initiated. Careful examination showed that Necl-1 and -4 are both located at the Schwann cell–axon interface, with Necl-1 on the axonal surface and Necl-4 on the adaxonal membrane of the Schwann cell. The Necl proteins are excluded from the nodes of Ranvier, but Necl-1, -2, and -4 are present in the juxtaparanodes, and Necl-4 is weakly expressed in the paranode. In addition, Necl-1, -2, and -4 are localized to Schmidt-Lanterman incisures, which are channels of cytoplasm that run through compact myelin.
Extensive biochemical studies show that Necl-4 on the Schwann cell and Necl-1 on the axon mediate axo–glial adhesion (Maurel et al., 2007; Spiegel et al., 2007). Binding assays with fusion proteins containing Necl ectodomains demonstrated a strong heterophilic interaction between Necl-1 and -4. In addition, Necl-1 and -2 also bind each other, and Necl-2 also exhibited weaker homophilic association. Moreover, purified Necl-1 and -2 can bind to Schwann cells, and Necl-4 and -2 can bind to neurites. Schwann cells could adhere to plastic surfaces coated with Necl-1 but not with Necl-2, suggesting that Necl-1 is more important for Schwann cell–axon interaction. Moreover, Necl-1 in neurons is required for the binding of a Necl-4 fusion protein, and knockdown studies also showed that Necl-4 in Schwann cells was required for Necl-1 binding (Maurel et al., 2007; Spiegel et al., 2007). The expression and biochemical studies together provide compelling evidence that heterophilic interaction between Necl-4 and -1 mediates adhesion between Schwann cells and axons along the internode (Maurel et al., 2007; Spiegel et al., 2007).
To test the functional roles of Necls on axons and glia, Maurel et al. (2007) used lentiviral vectors to target Necl-4 with short hairpin RNA. Knockdown of Necl-4 led to the dramatic inhibition of myelin segment formation in a myelinating culture system. Rescuing with a Necl-4 protein that was resistant to the short hairpin RNA not only restored myelination but actually increased the number of myelinated internodes. Myelination was also inhibited when the interaction between Necl-4 and -1 was disrupted by adding soluble Necl fusion proteins to myelinating cultures or injecting them into remyelinating nerves (Spiegel et al., 2007). Therefore, Necl-4 is required for myelination and may be present at limiting levels such that adding more increases the rate or amount of myelin formation in culture.
Collectively, these studies clearly show that Necls are important for Schwann cell–axon adhesion and myelination (Maurel et al., 2007; Spiegel et al., 2007). Intriguingly, interaction between Necl-4 and -1 is not required for Schwann cells to align with neurites (Maurel et al., 2007; Spiegel et al., 2007). These results together with the observation that ensheathing, nonmyelinating Schwann cells do not express Necl-4 (Spiegel et al., 2007) suggest that other molecules mediate adhesion between axons and developing Schwann cells and that the Necl proteins have a function that is specifically required for myelination to proceed. Consistent with this, the knockdown of Necl-4 reduced the expression of Oct-6, a transcription factor that acts at the promyelinating stage, and nearly eliminated Krox-20, which is required for myelination (Maurel et al., 2007). Thus, it is possible that Necls establish a specific interaction between premyelinating Schwann cells and axons and that this interaction is required for proper communication of the signals that trigger myelination.
The ectodomains of the Necls mediate adhesion, and it is likely that the cytoplasmic tails organize membrane-associated cytoskeletal and signaling complexes. A truncated construct containing the cytoplasmic tail of Necl-4 acted as a dominant-negative inhibitor of myelin formation (Spiegel et al., 2007). The exact role of the Necl cytoplasmic domains is unknown, but the presence of PDZ- and FERM-binding domains suggests several possibilities. A recent modeling study predicted that Necl-4 interacts with the PDZ domain of NHERF-2 (Stiffler et al., 2007), an adaptor that binds a variety of G protein–coupled and tyrosine kinase receptors (Weinman et al., 2006). This predicted interaction provides support for the notion that interaction between Necl proteins localizes receptors that are required for proper signaling. Another PDZ-containing protein with an interesting function in Schwann cell polarity and myelination is Par-3 (Chan et al., 2006). Like Necl-4, Par-3 is associated with the adaxonal Schwann cell membrane (Fig. 1), where it recruits the neurotrophin receptor p75. Schwann cell–axon contact establishes the asymmetric localization of Par-3, but it is not known how such contact recruits the Par-3 complex. Loss of Par-3 in myelinating cultures leads to a phenotype very similar to Necl-4 knockdown in which Schwann cells align with axons but fail to myelinate (Chan et al., 2006). Thus, it is possible that Necl interactions recruit Par-3 to the adaxonal surface, where it localizes molecular complexes that are required for signaling and myelination. The insightful analysis of Necl function in the peripheral nervous system greatly advances the understanding of the mechanisms that mediate axo–glial adhesion in the early stages of myelination (Maurel et al., 2007; Spiegel et al., 2007). Future work on these interesting proteins will determine how the Necl adhesion molecules organize Schwann cell–axon interaction and promote myelination.
References
- Chan, J.R., C. Jolicoeur, J. Yamauchi, J. Elliott, J.P. Fawcett, B.K. Ng, and M. Cayouette. 2006. The polarity protein Par-3 directly interacts with p75NTR to regulate myelination. Science. 314:832–836. [DOI] [PubMed] [Google Scholar]
- Fukuhara, H., M. Kuramochi, T. Nobukuni, T. Fukami, M. Saino, T. Maruyama, S. Nomura, T. Sekiya, and Y. Murakami. 2001. Isolation of the TSLL1 and TSLL2 genes, members of the tumor suppressor TSLC1 gene family encoding transmembrane proteins. Oncogene. 20:5401–5407. [DOI] [PubMed] [Google Scholar]
- Jessen, K.R., and R. Mirsky. 2005. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 6:671–682. [DOI] [PubMed] [Google Scholar]
- Kakunaga, S., W. Ikeda, S. Itoh, M. Deguchi-Tawarada, T. Ohtsuka, A. Mizoguchi, and Y. Takai. 2005. Nectin-like molecule-1/TSLL1/SynCAM3: a neural tissue-specific immunoglobulin-like cell-cell adhesion molecule localizing at non-junctional contact sites of presynaptic nerve terminals, axons and glia cell processes. J. Cell Sci. 118:1267–1277. [DOI] [PubMed] [Google Scholar]
- Maurel, P., S. Einheber, J. Galinska, P. Thaker, I. Lam, M.B. Rubin, S.S. Scherer, Y. Murakami, D.H. Gutmann, and J.L. Salzer. 2007. Nectin-like proteins mediate axon–Schwann cell interactions along the internode and are essential for myelination. J. Cell Biol. 178:861–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailov, G.V., M.W. Sereda, B.G. Brinkmann, T.M. Fischer, B. Haug, C. Birchmeier, L. Role, C. Lai, M.H. Schwab, and K.A. Nave. 2004. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 304:700–703. [DOI] [PubMed] [Google Scholar]
- Poliak, S., and E. Peles. 2003. The local differentiation of myelinated axons at nodes of Ranvier. Nat. Rev. Neurosci. 4:968–980. [DOI] [PubMed] [Google Scholar]
- Salzer, J.L. 2003. Polarized domains of myelinated axons. Neuron. 40:297–318. [DOI] [PubMed] [Google Scholar]
- Sherman, D.L., and P.J. Brophy. 2005. Mechanisms of axon ensheathment and myelin growth. Nat. Rev. Neurosci. 6:683–690. [DOI] [PubMed] [Google Scholar]
- Spiegel, I., K. Adamsky, M. Eisenbach, Y. Eshed, A. Spiegel, R. Mirsky, S.S. Scherer, and E. Peles. 2006. Identification of novel cell-adhesion molecules in peripheral nerves using a signal-sequence trap. Neuron Glia Biol. 2:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel, I., K. Adamsky, Y. Eshed, R. Milo, H. Sabanay, O. Sarig-Nadir, I. Horresh, S.S. Scherer, M.N. Rasband, and E. Peles. 2007. A central role for Necl4 (SynCAM4) in Schwann cell-axon interaction and myelination. Nat. Neurosci. 10:861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler, M.A., J.R. Chen, V.P. Grantcharova, Y. Lei, D. Fuchs, J.E. Allen, L.A. Zaslavskaia, and G. MacBeath. 2007. PDZ domain binding selectivity is optimized across the mouse proteome. Science. 317:364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai, Y., K. Irie, K. Shimizu, T. Sakisaka, and W. Ikeda. 2003. Nectins and nectin-like molecules: roles in cell adhesion, migration, and polarization. Cancer Sci. 94:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveggia, C., G. Zanazzi, A. Petrylak, H. Yano, J. Rosenbluth, S. Einheber, X. Xu, R.M. Esper, J.A. Loeb, P. Shrager, et al. 2005. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 47:681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voas, M.G., D.A. Lyons, S.G. Naylor, N. Arana, M.N. Rasband, and W.S. Talbot. 2007. alphaII-spectrin is essential for assembly of the nodes of Ranvier in myelinated axons. Curr. Biol. 17:562–568. [DOI] [PubMed] [Google Scholar]
- Weinman, E.J., R.A. Hall, P.A. Friedman, L.Y. Liu-Chen, and S. Shenolikar. 2006. The association of NHERF adaptor proteins with g protein-coupled receptors and receptor tyrosine kinases. Annu. Rev. Physiol. 68:491–505. [DOI] [PubMed] [Google Scholar]