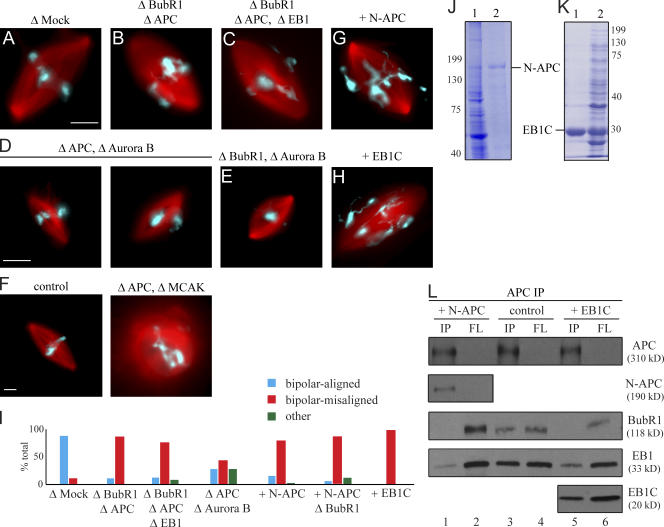
Figure 5.
BubR1–APC/EB1 complex formation is important for metaphase chromosome alignment. (A–H) Metaphase spindle structures assembled in mock- (A and E), BubR1/APC- (B), BubR1–APC/EB1- (C), APC/Aurora B- (D), BubR1/Aurora B- (E), and APC/MCAK (F) -depleted egg extracts or egg extracts supplemented with purified recombinant N-APC (G) or EB1C (H). (I) Quantification of structures formed from sperm nuclei in cycled egg extracts as indicated scored 80 min after exit from interphase. At least 50 mitotic structures were scored for each extract. Data are presented from one representative experiment. (J and K) Purification of recombinant Xenopus N-APC (APC1–1,450; J) and GST-EB1C (EB1165–268; K). Initial E. coli lysates encoding N-APC or EB1C (lane 1) and GST–N-APC or GST-EB1C after affinity purification over immobilized glutathione (lane 2). (L) Immunoprecipitates with an APC antibody from CSF-arrested egg extracts containing sperm nuclei (lanes 3 and 4) or supplemented with N-APC (lanes 1 and 2) or EB1C (lanes 5 and 6) with affinity-purified rabbit anti-APC IgG and probed with APC, BubR1, EB1, or GST (for GST–N-APC and GST-EB1C) antibodies. FL, extracts after immunoprecipitation; IP, immunoprecipitates. A threefold higher proportion of the bead-bound fraction relative to the depleted extracts was analyzed. Bars, 10 μm.