Figure 7.
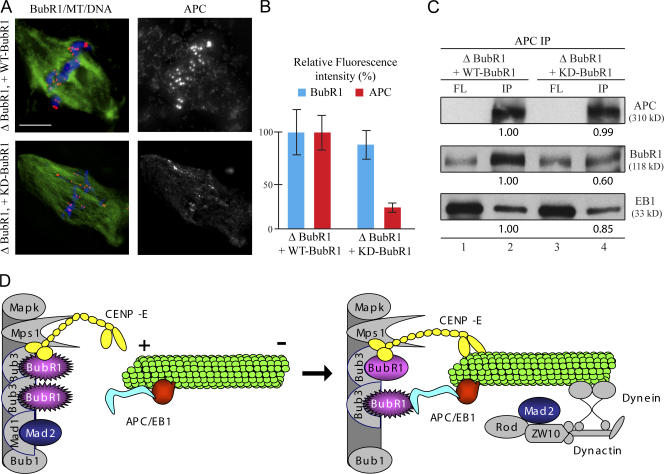
BubR1 kinase activity is essential for the efficient recruitment of APC onto kinetochores in Xenopus egg extracts. (A) BubR1-depleted egg extracts containing sperm nuclei were cycled through interphase and arrested with CSF activity at the following metaphase. Recombinant WT-BubR1 (top) or KD-BubR1 (bottom) was added. Kinetochore recruitment of BubR1 (left; red), APC (right), and microtubules (left; green) was visualized by immunofluorescence with specific antibodies. Chromatin was visualized with DAPI (left; blue). (B) Quantification of the relative BubR1 and APC intensity at kinetochores in A. Error bars represent SEM. (C) Immunoprecipitates with an APC antibody from BubR1-depleted CSF egg extracts containing sperm nuclei and supplemented with purified WT-BubR1 (lanes 1 and 2) or KD-BubR1 (lanes 3 and 4) and probed with APC (top), BubR1 (middle), and EB1 (bottom) antibodies. FL, extracts after immunoprecipitation; IP, immunoprecipitates. A threefold higher proportion of the bead-bound fraction relative to the depleted extracts was analyzed. (D) Model: BubR1 is recruited onto unattached kinetochores. Microtubule-associated proteins APC/EB1 bind to the plus ends of microtubules. After the initial capture of microtubules by kinetochores, the interaction between BubR1 and APC/EB1 stabilizes kinetochore microtubule attachment, in which BubR1 might directly phosphorylate APC. Bar, 10 μm.