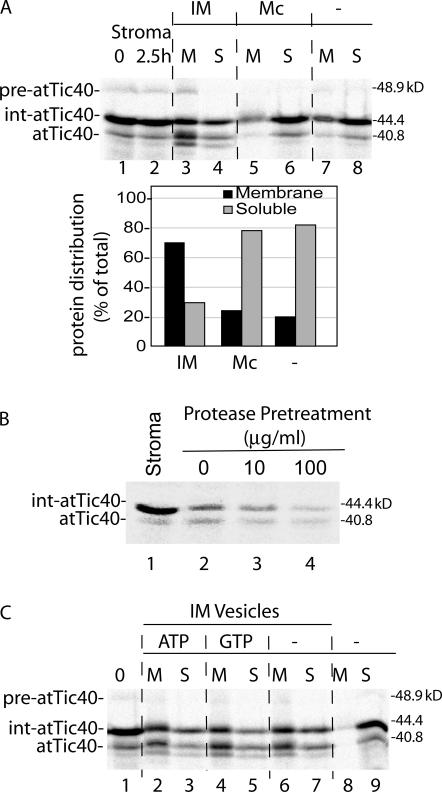
Figure 8.
The insertion of int-atTic40 into IM vesicles is selective and requires proteinaceous components at the membrane. (A) Int-atTic40 does not bind to canine pancreatic microsomes. A stromal extract containing [35S]int-atTic40 was incubated in the absence (−) or presence of isolated IM vesicles (30 μg protein) or canine pancreatic microsomes (Mc; 30 μg protein) for 2.5 h. After the reaction, the samples were separated into membrane (M) and soluble (S) fractions. The quantitative analysis presents the distribution of combined [35S]-labeled int-atTic40 and atTic40 between the membrane and soluble fractions from each reaction. (B) Int-atTic40 insertion into IM vesicles relies on protease-sensitive membrane components. Isolated IM vesicles were treated with the indicated concentrations of thermolysin before incubation with stromal extract containing [35S]int-atTic40. The fraction of [35S]int-atTic40 that associated with the membranes after alkaline extraction is shown in lanes 2–4. Lane 1 contains a sample of stromal extract equivalent to that added to each reaction. (C) Int-atTic40 targeting to IM vesicles does not require exogenous nucleoside triphosphates. A dialyzed stromal extract containing [35S]int-atTic40 was incubated with IM vesicles in the absence (−) or presence of 2 mM ATP or GTP as indicated. The reactions were separated into membrane and soluble fractions by alkaline extraction and analyzed by SDS-PAGE and phosphorimaging. Lane 1 contains a sample of stromal extract equivalent to that added to each reaction. Lanes 8 and 9 contains stromal extract that was incubated in the absence of IM vesicles and subsequently treated with alkaline buffer.