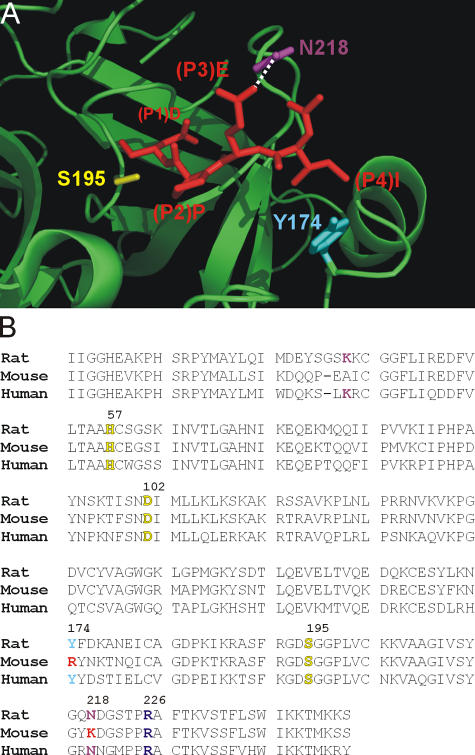
Figure 4.
Structural differences in the S4 and S3 subsites of human and mouse GrB. (A) Interaction of the IEPD peptide with the active site cleft of hGrB. Shown is a section of the structure of hGrB (green ribbon) complexed to the inhibitor IEPD-CHO (red stick; available from Protein Data Bank under accession no. 1IAU; Rotonda et al., 2001). Highlighted are the GrB residues Y174 and N218 predicted to interact with the P4 and P3 residues of the peptide. The active site serine (S195) is also indicated. (B) Alignment of the human, mouse, and rat GrB sequences. This illustrates that Y174 and N218 are not conserved in the mouse. R226 (shown in purple), which is at the bottom of the S1 pocket and interacts with P1 Asp, is conserved. Catalytic triad residues are indicated in yellow.