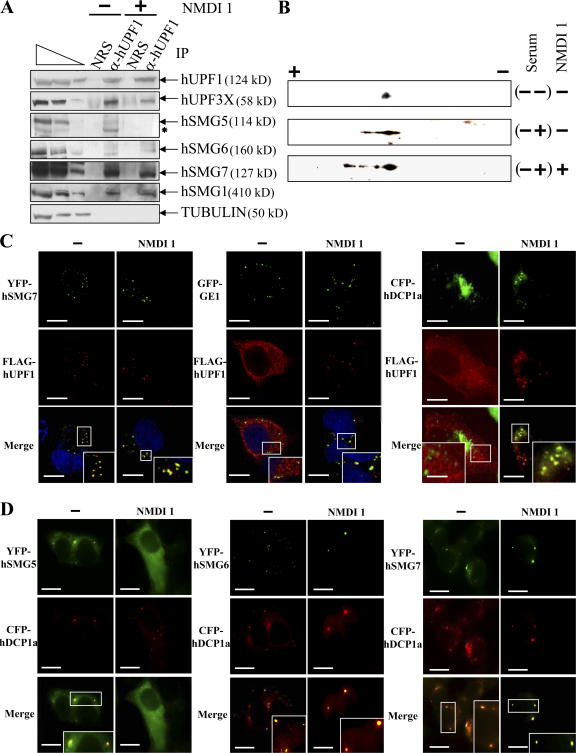
Figure 3.
NMDI 1 modifies the cellular distribution of hUPF proteins and stabilizes hyperphosphorylated isoforms of FLAG-hUPF1. (A) Endogenous hUPF1 protein was immunopurified using rabbit anti-hUPF1 antibodies from HeLa cell extracts that were incubated with DMSO(−) or 5 μM NMDI 1(+). In parallel, an immunopurification control was performed using normal rabbit serum to verify the specificity of the immunopurification protocol. The three leftmost lanes correspond to serial twofold dilutions of a whole HeLa cell extract. The asterisk marks an uncharacterized band that presumably represents a degradation product. (B) 2D gel analysis of the FLAG-hUPF1 phosphorylation level. 106 293T cells were transfected with 1 μg pCI-neo-FLAG-hUPF1 plasmid. 12 h later, the serum was removed from the culture medium for 24 h, and then either DMSO or 5 μM NMDI 1 was added to the culture medium for 3 h before adding back 10% serum (sample (−+) except in sample (−−)). Proteins were extracted and loaded on a 2D gel analysis system (see Materials and methods). (C) Immunofluorescence assay. HeLa cells were transfected with pCI-neo-FLAG-hUpf1 and either pYFP-hSmg7, pGFP-Ge1, or pCFP-hDcp1a. 24 h after transfection, cells were incubated with DMSO(−) or 5 μM NMDI 1 for 20 h. The blue staining visible in the merge of the two leftmost set of images corresponds to nuclei staining by Hoechst stain. (D) Immunofluorescence assay. HeLa cells were transfected with pYFP-hSmg5, pYFP-hSmg6, or pYFP-hSmg7 and with pCFP-hDcp1a expression vectors. 24 h after transfection, cells were cultured in the presence of DMSO(−) or 5 μM NMDI 1 for 20 h. The white squares are magnifications of cell areas. Bars, 10 μm.