Figure 1.
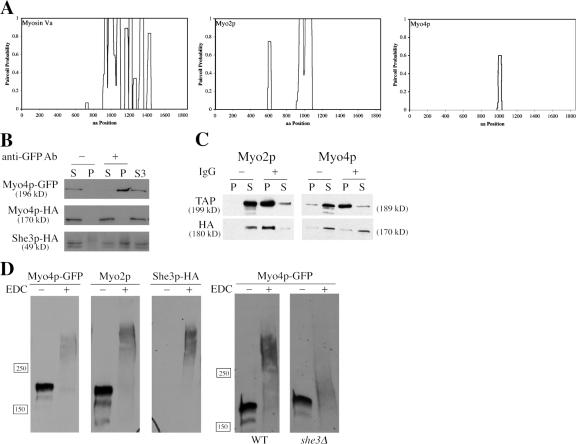
Myo4p is a monomeric, class V myosin in cell extracts. (A) Paircoil predictions for myosin Va, Myo2p, and Myo4p. Myo4p has a predicted coiled-coil domain of only 30 amino acids, considerably shorter than predicted coiled-coil domains in myosin Va or Myo2p. (B) Myo4p-HA does not coprecipitate with Myo4p-GFP. MYO4-HA was expressed on a CEN/ARS plasmid in YPT87. Myo4p-GFP was immunoprecipitated from yeast lysates, and supernatants and pellets were subsequently analyzed by Western blot. Myo4p-HA was not detected in the Myo4p-GFP immunoprecipitate, but She3p-HA was clearly present. S3 is a high-speed supernatant and represents the starting material for the immunoprecipitations. (C) Myo2p-HA coprecipitates with Myo2p-1/2TAP. MYO2-HA and MYO4-HA were expressed on CEN/ARS plasmids in YPT117 and YPT118, respectively. Myo2p-1/2TAP and Myo4p-1/2TAP were immunoprecipitated from cell lysates with IgG beads, and supernatants and pellets were subsequently analyzed by Western blot. Myo2p-HA was evident in immunoprecipitates of Myo2p-Tap1/2, but Myo4p-HA was not detected in Myo4p-Tap1/2 immunoprecipitates. (D) Chemical cross-linking indicates that Myo4p is a monomer. Cell lysates from YPT68 and YPT70 were treated with 50 mM EDC for 1 h at RT and then analyzed by Western blot. EDC-treated Myo4p-GFP migrated as a larger complex than untreated Myo4p-GFP, but smaller than EDC-treated Myo2p. Note that a single Myo4p-GFP polypeptide is ∼15 kD larger than a single Myo2p polypeptide, so a cross-linked Myo4p-GFP dimer would be expected to be larger than a cross-linked Myo2p dimer. She3p-HA cross-links with Myo4p-GFP. EDC-treated Myo4p-GFP in she3Δ extracts showed a minimal shift in size compared with untreated Myo4p-GFP.