Figure 3.
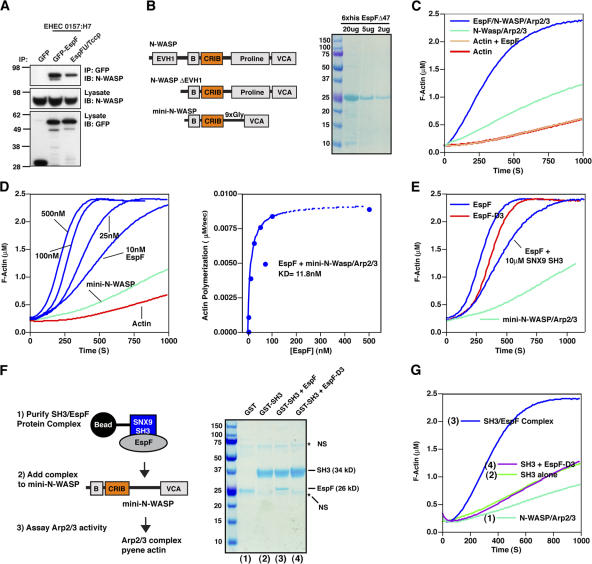
EspF directly binds to and activates N-WASP. (A) HEK293A cells were cotransfected with EGFP-EspF, EGFP-EspFu/TccP, or control EGFP and V5-tagged N-WASP. Anti-GFP immunoprecipitations (IP) were probed by V5 immunoblot (IB) (top). Cell lysates were probed by V5 or GFP immunoblot to show input levels (bottom two panels). (B) A diagram depicting N-WASP ΔEVH1 and mini-N-WASP proteins used in actin polymerization experiments and a Coomassie-stained gel of purified 6×His EspFΔ47 (residues 48–206) used in the actin polymerization experiments. (C) Pyrene-actin assembly assay demonstrating EspF activates N-WASP in vitro. The polymerization kinetics of actin alone (red) was not increased by addition of 500 nM EspF (gold). Polymerization curves of Arp2/3 and N-WASPΔEVH1 (light green) compared with these components plus 500 nM EspF (blue) is shown. Unless otherwise stated, all assays contain 2.5 μM pyrene-actin, 40 nM Arp2/3 complex, and 100 nM N-WASP proteins. (D) EspF activates mini-N-WASP in vitro. A pyrene-actin assembly assay showing that EspF activated mini-N-WASP in a dose-dependent manner (blue). The rate of actin polymerization for each EspF concentration was determined at 2 μM G-actin consumption (80%) and plotted against EspF protein concentration (right graph). (E) Pyrene actin assembly assays comparing EspF (100 nM) and mutant EspF-D3 (100 nM) activating mini-N-WASP. Addition of 10 μM GST-SNX9 (SH3) domain to EspFΔ47 had a negligible affect on mini-N-WASP activation. (F) Schematic depicting the experimental procedure for Fig. 3 G (left) and an SDS-PAGE of GST (1), GST-SNX9-SH3 (2), GST-SNX9-SH3 in complex with EspFΔ47 (3), and GST-SNX9-SH3 control that did not form a complex with mutant EspF-D3 (4). The mobility of the stable SH3/EspFΔ47 complex is indicated. Non-specific (NS) bands are indicated (*). (G) Mini-N-WASP actin polymerization assay on protein complexes described in Fig. 3 F.