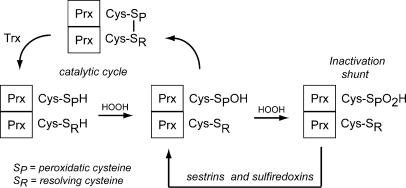
Figure 1.
The catalytic cycle of C-Cys eukaryotic Prxs. (A) When exposed to H2O2, the peroxidatic cysteine (SPH) of 2-Cys Prxs is oxidized to sulfenic acid (Prx-SOH). Upon reaction with the resolving cysteine (SRH), a Prx dimer with an intermolecular disulfide bond is formed, which is then reduced by Trx to regenerate active enzyme. Because of a pause in the catalytic cycle, the SPH of eukaryotic 2-Cys Prxs is susceptible to hyperoxidation, resulting in the formation of a sulfinic acid form (Prx-SO2H) that is catalytically inactive. Sulfiredoxins and sestrins are ATP-dependent sulfinyl reductases that participate in retroreduction of Prx-SO2H, regenerating active enzyme. 2-Cys Prxs are obligate homodimers that can assemble into decamers and higher molecular mass oligomers, depending on oxidation state, pH, calcium concentrations, and posttranslational modifications such as phosphorylation.