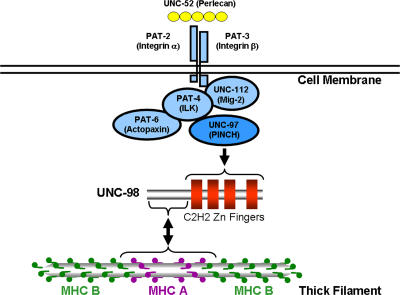
Figure 5.
UNC-98 as a molecular bridge between integrin-associated proteins and thick filaments at the M-line. In C. elegans body wall muscle, the myofibrils are closely apposed to the muscle cell membrane, and both the dense bodies (Z-disks) and M-lines are attached to the muscle cell membrane. At the base of both these focal adhesion–like structures are clustered UNC-52 (perlecan) in the ECM and the integrins in the muscle cell membrane. Associated with the cytoplasmic tail of PAT-3 is a complex of four proteins, including UNC-97 (PINCH). At the M-line, UNC-97 interacts with the four C2H2 Zn fingers of UNC-98, whereas the N terminus of UNC-98 interacts with the C-terminal portion of MHC A, but not MHC B. In nematode thick filaments, these MHCs are differentially localized, with MHC A in the middle and MHC B in the polar regions. An arrow points from UNC-97 to UNC-98 because, when the level of UNC-97 is reduced, UNC-98 is mislocalized. The arrow points in both directions between UNC-98 and MHC A because, in the absence of MHC A, UNC-98 is found in large aggregates. When UNC-98 levels are reduced, MHC A is not localized in its normal sharply defined pattern.