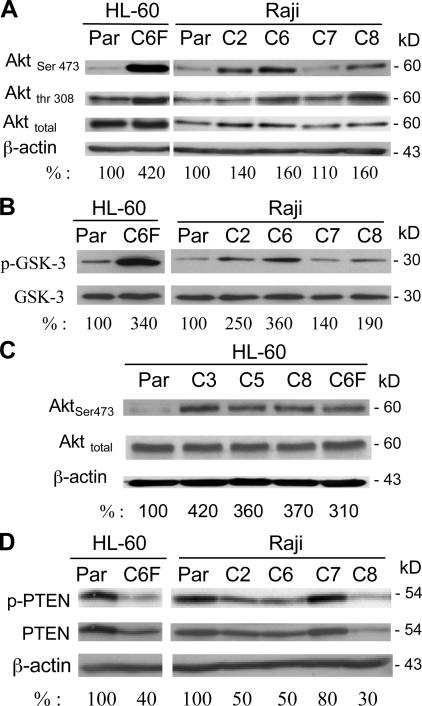
Figure 3.
The Akt pathway is constitutively activated in ρ- cells. (A) Akt protein expression and its phosphorylation status were determined using anti-Akt and anti–phospho-Akt (Ser-473 and Thr-308) antibodies. The numbers below each lane indicate the relative density. (B) Comparison of in vitro kinase activity of protein extracts from ρ- clones cells and the parental cells. Akt protein was first immunoprecipitated with an anti-Akt antibody, and Akt enzyme activity was determined using an Akt kinase assay kit with GSK-3α/β fusion protein as the assay substrate, as described in Materials and methods. (C) Activation of Akt in ρ- cells from HL-60. Akt protein expression and its active phosphorylation were determined using anti-Akt and anti–phospho-Akt (Ser-473) antibodies. Whole-cell extracts from the parental HL-60 cells and four ρ- clones were prepared and subjected to immunoblotting analysis. (D) Reduced PTEN activity in the respiration-deficient ρ- clones. Expression of both activated PTEN (phosphorylated; phospho-PTEN) and total PTEN protein were determined using anti–phospho-PTEN antibodies (Ser-380 and Thr-382/383) and anti-PTEN antibody. The numbers below each lane show the relative band density normalized by β-actin. The value for the parental sample was expressed as 100%.