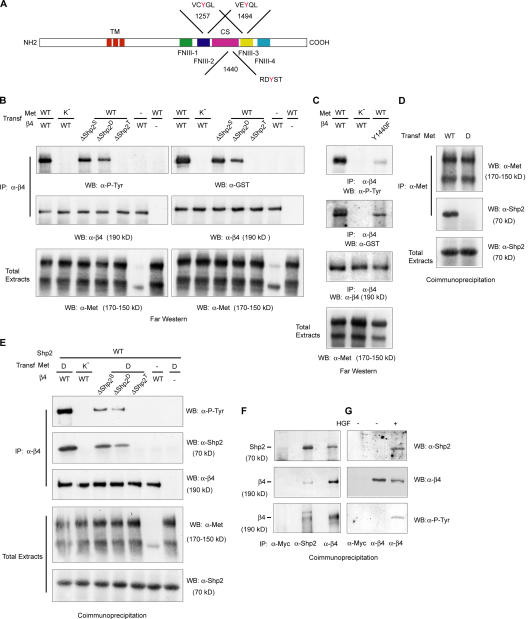
Figure 1.
Tyrosine-phosphorylated β4 interacts with Shp2. (A) Schematic representation of β4 structure. The cytoplasmic domain contains two pairs of type III fibronectin-like repeats (FNIII) separated by a connecting segment (CS). The consensus sequences for Shp2 and tyrosine positions are indicated. TM, transmembrane domain. (B) β4–Shp2 association by far-Western analysis. COS-7 cells were cotransfected with wild-type (WT) or kinase-dead (K−) Met and with wild-type β4 or β4 mutants bearing single (β4-ΔShp2S; Y1257F), double (β4-ΔShp2D; Y1257F and Y1494F), and triple (β4-ΔShp2T; Y1257F, Y1440F, and Y1494F) phenylalanine substitutions of the potential docking tyrosines for Shp2. Lysates from each experimental point were subdivided into two aliquots and immunoprecipitated (IP) in parallel with antibodies to β4; Western blots (WB) were probed with antiphosphotyrosine (P-Tyr) antibodies (left) or with a GST-Shp2 fusion protein followed by anti-GST antibodies (right). The blots were then reprobed with anti-β4 antibodies. A fraction of the lysates (1/50) was loaded to detect Met from total extracts. In COS-7 cells, exogenous Met is expressed as both the precursor (top band) and mature form (bottom band). The weak band observable in the sixth lane corresponds to endogenous Met. (C) A β4 mutant with a single phenylalanine permutation of Tyr1440 (β4-Y1440F) displays reduced but detectable tyrosine phosphorylation and can bind Shp2 in far-Western analysis. (D) Coimmunoprecipitation experiment showing that wild-type Met but not a Met mutant with phenylalanine substitutions of the two tyrosines of the multidocking site (MetD) associates with Shp2. (E) Coimmunoprecipitation of β4 and Shp2 from lysates of COS-7 cells overexpressing Shp2 and the indicated forms of Met and β4. (F and G) Coimmunoprecipitation of β4 and Shp2 in cells with the endogenous expression of both proteins. (F) Reciprocal coimmunoprecipitation experiment in GTL16 cells. Cell lysates were immunoprecipitated with antibodies to myc (negative control), Shp2 (positive control), or β4. Blots were probed with antibodies against Shp2 (top), β4 (middle), or phosphotyrosine (P-Tyr; bottom). Shp2 is present in β4 immunoprecipitates (top), and β4 is present in Shp2 immunoprecipitates (middle). Immunopurified β4 and Shp2-associated β4 appear to be constitutively tyrosine phosphorylated. (G) FG2 cells were either left untreated or were treated with 100 ng/ml HGF for 10 min. Lysates were immunoprecipitated with antibodies to myc or β4, and blots were probed as indicated. Shp2 can be recovered from β4 immunoprecipitates after HGF stimulation (top). Treatment with HGF induces the tyrosine phosphorylation of β4 (bottom).