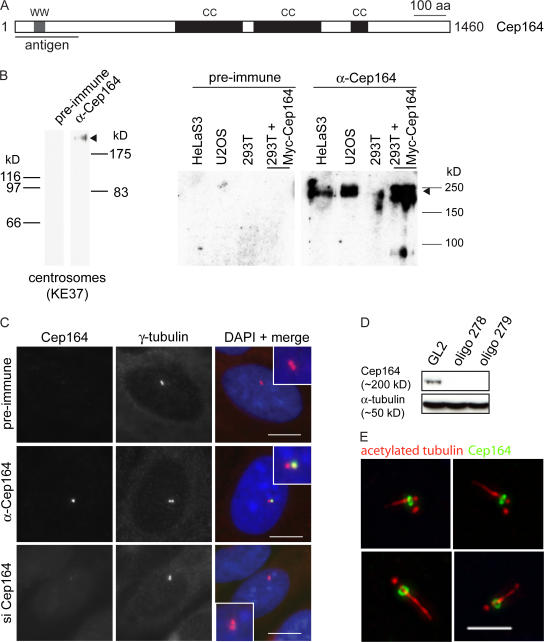
Figure 2.
Characterization of anti-Cep164 antibodies. (A) Schematic illustrating primary structure of human Cep164. The protein is predicted to comprise an N-terminal WW domain (WW) and three coiled-coil domains (CC). The horizontal line underneath the scheme denotes the region used for antibody production. (B) Antibodies directed against Cep164 and corresponding preimmune serum were tested by Western blotting on centrosome preparations from KE37 cells (left) and total lysates of HeLaS3, U2OS, and 293T cells as well as 293T cells overexpressing myc-Cep164 (right). Affinity-purified antibody was used at 1 μg/ml. Arrowhead indicates Cep164. Note that in the centrosome sample Cep164 shows a retarded electrophoretic mobility caused by a high sucrose concentration. (C) Antibodies directed against Cep164 and corresponding preimmune serum were used in IF microscopy on U2OS cells. The anti-Cep164 antibody (green) recognizes only one of two centrioles, as indicated by colocalization with γ-tubulin (red). This staining was abolished upon siRNA-mediated depletion of Cep164, and preimmune serum showed no specific staining. (insets) Enlarged centrosome areas. (D) Western blot on U2OS cells illustrating efficient depletion of Cep164 by siRNA. Transfections were performed for 48 h using two different Cep164-specific oligonucleotide duplexes (278 and 279) or GL2 for control. Blotting for α-tubulin is shown to control for equal loading. (E) Cep164 localizes to mature centrioles at the base of the PC but not along the length of the axoneme. hTERT-RPE1 cells were serum starved for 48 h to induce PC formation and stained with antibodies against Cep164 (green) and acetylated tubulin (red). The four representative examples show bar- and ringlike staining of Cep164 at the base of the PC in close association with the mature centriole. Bars: (C) 10 μm; (E) 5 μm.