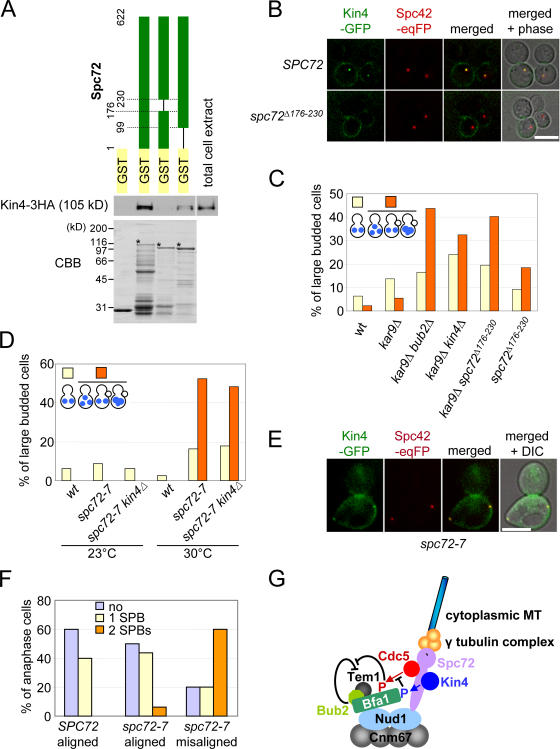
Figure 7.
A role of the γ-tubulin complex receptor protein Spc72 in regulation of the SPOC. (A) Kin4 binds to the SPB component Spc72. Kin4 pull-down assays with recombinant GST, GST-Spc72, GST-Spc72Δ176–230, and GST-Spc72Δ1–98. Total cell extract prepared from a yeast strain carrying KIN4-3HA was incubated with the purified recombinant proteins bound to glutathione beads (CBB-stained gel). After washing of the beads, Kin4-3HA was detected by immunoblotting. Asterisks mark the full-length Spc72 proteins. (B) In spc72 Δ176–230 cells, Kin4-GFP fails to bind to SPBs. SPC72 and spc72 Δ176–230 cells with KIN4-GFP SPC42-eqFP611 were treated with nocodazole. Kin4-GFP localization was determined by fluorescence microscopy. (C) spc72 Δ176–230 cells are SPOC deficient. Wild-type (wt), kar9Δ, kar9Δ bub2Δ, kar9Δ kin4Δ, kar9Δ spc72Δ 176–230, and spc72 Δ176–230 cells were grown to mid-log phase in YPAD at 30°C and were shifted to 37°C for 3 h. Cells were fixed, and the DNA was stained with DAPI. Large-budded cells of wild-type, kar9Δ, kar9Δ bub2Δ, kar9Δ kin4Δ, kar9Δ spc72 Δ176–230, and spc72 Δ176–230 cells were 27.9%, 33.1%, 42.0%, 29.5%, 35.8%, and 33.0%, respectively. n > 100 large-budded cells per strain. (D) spc72-7 cells are SPOC deficient. Wild-type (wt), spc72-7, and spc72-7 kin4Δ cells were grown at 23 and 30°C to mid-log phase. Cells were fixed, and DNA was stained with DAPI. >200 cells per strain. Large-budded cells of wild-type, spc72-7, and spc72-7 kin4Δ cells were 23.8%, 27.4%, and 31.8% at 23°C and 24.0%, 49.2%, and 47.3% at 30°C, respectively. (E) Kin4-GFP binds to SPBs in spc72-7 cells. SPC72 and spc72-7 cells with KIN4-GFP SPC42-eqFP611 were synchronized with α factor at 23°C and released at 30°C. Anaphase cells were analyzed. (F) Quantification of E. n > 30 anaphase cells per strain. (G) Model for the function of Kin4. See Discussion for details. Bars, 5 μm.