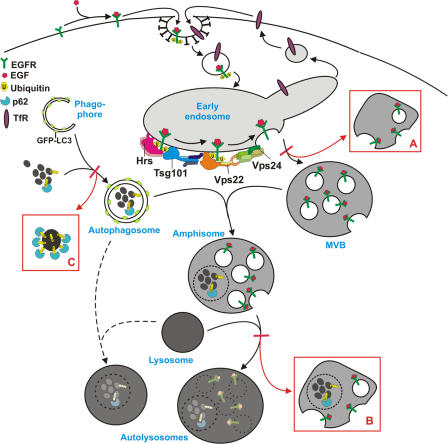
Figure 10.
Model for autophagic degradation in control and ESCRT-depleted cells. In control cells, cytoplasmic cargo (proteins and organelles) is sequestered by an isolation membrane/ phagophore, forming double-membrane autophagosomes that can fuse with MVBs, forming amphisomes. Amphisomes, containing both endocytic and autophagic cargo, then fuse with lysosomes, forming autolysosomes, where the content is degraded. Autophagosomes may also fuse directly with lysosomes, although the amphisome pathway seems to be the major pathway in HeLa cells. The ESCRT complexes are required for proper sorting and degradation of ubiquitinated integral membrane proteins (e.g., EGFR) and for proper MVB morphology, and depletion of ESCRT subunits results in the formation of aberrant MVBs (morphology depending of which ESCRT subunit is depleted) (red box A). Degradation of autophagic cargo is also inhibited in ESCRT-depleted cells, proposedly because of inhibited formation of autolysosomes (red box B), although autophagosomes and amphisomes are still formed. In addition, large p62- and ubiquitin-positive membrane-free aggregates accumulate in ESCRT- depleted cells (red box C), indicating that continuous autophagic clearance of cytoplasmic proteins is important to avoid accumulation of ubiquitin-positive aggregates that may cause neurodegeneration.