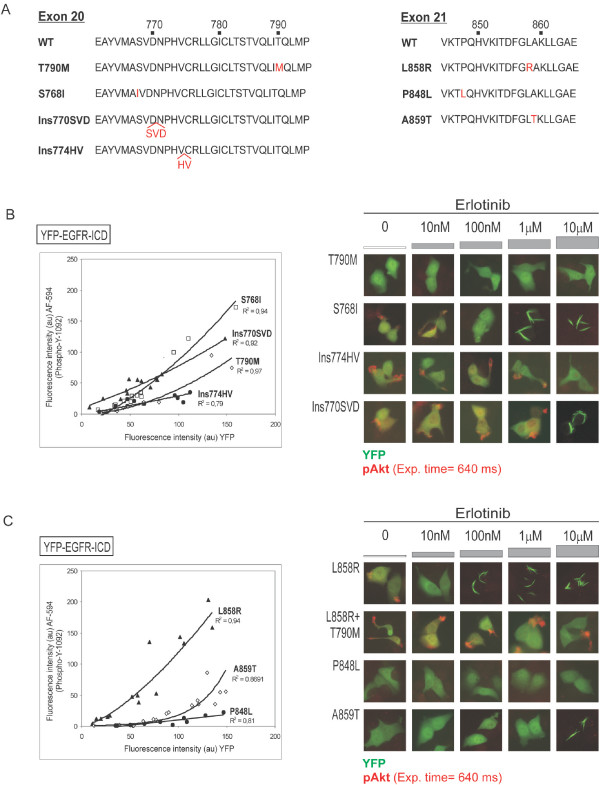
Figure 4.
Testing the kinase activity and erlotinib sensitivity of uncommon EGFR mutants using the YFP-EGFR-ICD assay. A. Partial amino acid sequence of EGFR exon 20 and exon 21 illustrating the location of the mutations examined (red letters). B. Kinase activity and erlotinib sensitivity of different exon 20 mutations. Graph shows that autophosphorylation levels are lower for T790M (white diamonds) than for S768I (white squares) or Ins770SVD (black triangles). Low expression levels hampered the accurate evaluation of Ins774HV (black circles). Images show that YFP-EGFR T790M did not effectively induce phosphorylation of endogenous Akt in MCF-7 cells, and did not relocate into fibrils upon erlotinib treatment. S768I-induced pAkt was inhibited by 100 nM erlotinib and the ectopic protein relocated into fibrils at 1 μM. The phosphorylation of Akt induced by exon 20 insertions was only inhibited at 10 μM erlotinib. This drug concentration also induced relocation of YFP-EGFR-ICD Ins770SVD into fibrils. C. Kinase activity and erlotinib sensitivity of different exon 21 mutations. Graph shows that the common L858R mutation confers higher autophosphorylation levels to YFP-EGFR-ICD than P848L and A859T. Images show that, unlike L858R, these uncommon exon 21 mutants did not induce phosphorylation of endogenous Akt. Erlotinib blocked L858R-induced pAkt at 10 nM, and caused relocation of the ectopic protein into fibrils at 100 nM. Both effects were readily abrogated by the TKI-resistant mutation T790M. In all cases, data corresponding to one experiment are shown. Each EGFR mutant was tested at least twice with similar results.