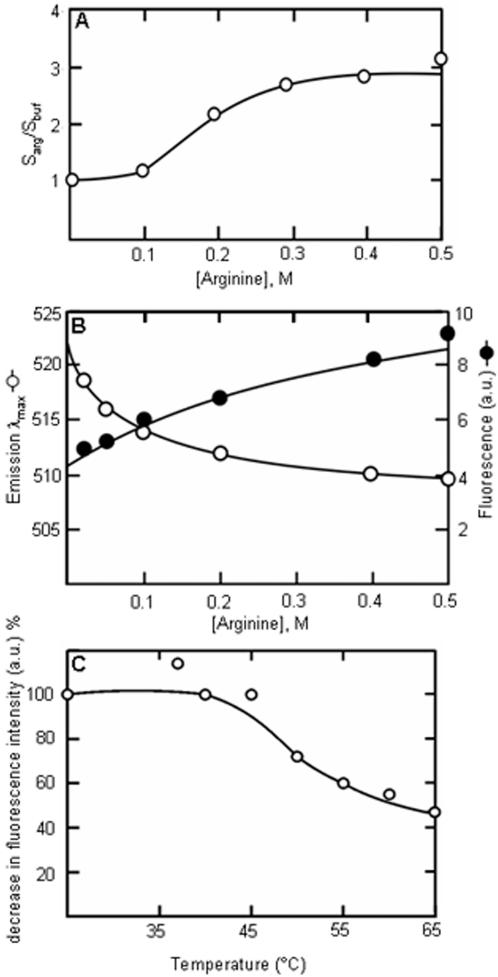
Figure 1. Non-polar environment in arginine solutions.
(A) Pyrene solubility in presence of arginine. The solubility is expressed as fold-increase over the control (solubility in arginine/solubility in buffer). 1 mg pyrene was incubated in arginine solutions at the indicated concentrations at 25°C for 24 h. The absorbance of the supernatant solution was measured at 350 nm. The solubility increases in a dose dependent manner. (B) ANS fluorescence in the presence of arginine. The excitation wavelength was 400 nm and the emission intensity was scanned from 450 to 600 nm. With the increase in arginine concentration, the maximum emission wavelength of ANS (250 µM in PB) decreases (open circle) and relative fluorescence intensity increases (closed circle). (C) Temperature dependence of ANS fluorescence in the presence of 0.2 M arginine. The observed intensity is expressed as % of intensity at 25°C. The intensity decreases above 45°C.