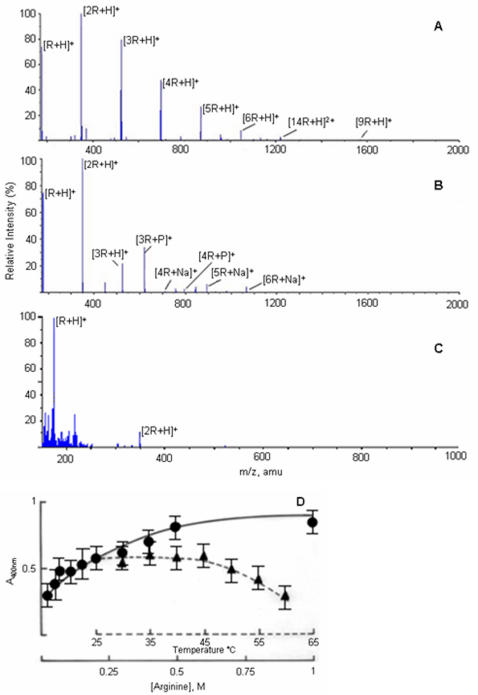
Figure 2. Molecular clusters of arginine in solution.
Electrospray mass spectroscopy of amino acids. The aqueous solutions amino acids at 0.2 M concentrations were used. (A) Arginine exhibits extensive noncovalent protonated clusters when dissolved in water, pH without adjustment was 10.5. (B) ([nArg]Na)+ and ([nArg]H2PO4)+ clusters are observed when arginine is dissolved in sodium phosphate buffer, pH 7.4. (C) Less extensive clustering is seen in acidic solutions at pH 1.0. (D) Increase in Rayleigh light scattering by arginine solution (in PB) is concentration dependent (filled circle) indicating supramolecular assembly. This assembly is temperature sensitive and collapses above 45°C (filled triangle).