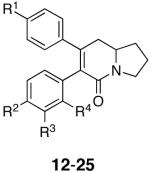
Table 1.
| Analog | Northern Aryl Substitution R1 | Southern Aryl Substitution R2, R3, R4 | HCT-116 Cytotoxicity IC50, μM |
|---|---|---|---|
| 12 | OMe | H, H, H | 0.39 |
| 13 | OMe | Cl, H, H | 13 |
| 14 | OMe | OMe, H, H | 3.2a |
| 15 | OMe | Me, H, H | 10 |
| 16 | OMe | Cl, Cl, H | 4 |
| 17 | OMe | H, Cl, H | 8 |
| 18 | OMe | H, H, Cl | 8 |
| 19 | OMe | H, NH-LCBb, H | >25 |
| 20 | OMe | NH-LCBb, H, H | 25 |
| 21 | OMe | N3, H, H | 25 |
| 22 | O-LCBb | H, H, H | 17 |
| 23 | OMe | H, NH2, H | >25 |
| 24 | OH | H, H, H | 9 |
| 25 | OBn | H, H, H | 8.9 |
Assay conducted by National Cancer Institutes Developmental Therapeutics Program.
LCB = (+)-biotinyl-6-aminohexanoic acid.
The cytotoxicity experiments were done as previously described32 except that the cells were incubated for 48 h after the addition of the compounds.