Abstract
Background
The traditional recommendations which suggest that hypotonic intravenous (IV) maintenance fluids are the solutions of choice in paediatric patients have not been rigorously tested in clinical trials, and may not be appropriate for all children.
Aims
To systematically review the evidence from studies evaluating the safety of administering hypotonic versus isotonic IV maintenance fluids in hospitalised children.
Methods
Data sources: Medline (1966–2006), Embase (1980–2006), the Cochrane Library, abstract proceedings, personal files, and reference lists. Studies that compared hypotonic to isotonic maintenance solutions in children were selected. Case reports and studies in neonates or patients with a pre‐existing history of hyponatraemia were excluded.
Results
Six studies met the selection criteria. A meta‐analysis combining these studies showed that hypotonic solutions significantly increased the risk of developing acute hyponatraemia (OR 17.22; 95% CI 8.67 to 34.2), and resulted in greater patient morbidity.
Conclusions
The current practice of prescribing IV maintenance fluids in children is based on limited clinical experimental evidence from poorly and differently designed studies, where bias could possibly raise doubt about the results. They do not provide evidence for optimal fluid and electrolyte homoeostasis in hospitalised children. This systematic review indicates potential harm with hypotonic solutions in children, which can be anticipated and avoided with isotonic solutions. No single fluid rate or composition is ideal for all children. However, isotonic or near‐isotonic solutions may be more physiological, and therefore a safer choice in the acute phase of illness and perioperative period.
Keywords: intravenous fluids, hypotonic, isotonic, hyponatraemia
Intravenous (IV) maintenance fluids are designed to provide free water and electrolyte requirements in a fasting patient. The prescription for IV maintenance fluids was originally described in 1957 by Holliday and Segar, who equated free water requirements from energy expenditure in healthy children.1 They rationalised adding 3.0 and 2.0 mEq/100 kcal/24 h of sodium and potassium respectively, as it approximates the electrolyte requirements and urinary excretion in healthy infants.2,3 This is the basis for the current recommendation that hypotonic IV maintenance solutions are ideal for children.4,5 The Holliday–Segar system remains the most universally used to date, because of the simplicity of their formula. While these recommendations may be appropriate for the healthy child, they do not necessarily apply in acute illness, where energy expenditure and electrolyte requirements deviate significantly from this formula.6
The numbers of deaths and significant neurological sequelae from hospital acquired hyponatraemia in children receiving hypotonic maintenance solutions have increased in the past 10 years.7,8,9,10,11 Several narrative reviews have suggested potential harm with these solutions and recommend that routine use in children be reconsidered.12,13 Despite these concerns, standard texts and guidelines continue to recommend hypotonic maintenance solutions for all paediatric patients.4,5 The objective of this systematic review was to evaluate the safety of hypotonic versus isotonic IV maintenance solutions in hospitalised children. Our secondary objective was to identify subgroups who are at greater risk of morbidity, in whom hypotonic solutions should be avoided.
Methods
Search strategy
We searched Medline (1966–2006), Embase (1980–2006), and the Cochrane Library, using the terms: “fluid therapy”, “hypotonic solution”, “isotonic solution”, and synonyms or related terms (Appendix 1; see http://www.archdischild.com/supplemental). We searched online (FirstSearch, Conference Proceedings) or published conference proceedings, and Current Controlled Trials (www.controlled‐trials.com). Abstracts from the following 2002–05 scientific forums were hand searched: World Congress on Pediatric Intensive Care, Society for Pediatric Research, Critical Care Congress, and American Academy of Pediatrics. We reviewed the reference lists of all identified studies and reviews, and also personal files, and contacted experts and first authors to identify other published or unpublished studies.
Study selection
Citations considered potentially relevant by either of two reviewers (KC or MK) were retrieved using the following inclusion criteria:
Controlled trials, cohort, and case‐control studies. Cohort studies had to compare patients receiving hypotonic IV maintenance solutions with a control group or unexposed cohort who received isotonic solutions. Case‐control studies had to compare cases, to a control group who did not have the outcomes of interest.
Children (1 month to 17 years) hospitalised for any medical or surgical condition. We included a diverse paediatric population to capture all potential patients who currently receive “standard IV maintenance therapy”.
Intervention: currently used hypotonic and isotonic IV maintenance solutions. Solutions were classified as “hypotonic” if they contained <0.9% NaCl, or “isotonic or near isotonic” (i.e. 0.9% NaCl or Ringers Lactate). We excluded case reports and studies of fluid resuscitation and oral rehydration therapy. Studies enrolling neonates, patients with pre‐existing hyponatraemia and co‐morbidities which result in sodium derangements (e.g. renal disease, diabetes insipidus, diuretic therapy), were also excluded.
Study outcomes
Studies were included if any of the following outcomes related to the development of acute hospital acquired plasma sodium (PNa) derangements and/or their attributed morbidity were reported: fluid balance, clinical evidence of volume overload, hypertension, seizures, cerebral oedema, death, paediatric intensive care unit admission, and length of stay. We used PNa as a surrogate outcome, as it is a convenient reflection of tonicity balance, and represents the potential for fluid shifts between intracellular and extracellular fluid (ECF) compartments. This in turn may result in clinically relevant morbidity, such as the defined outcomes of interest. A priori, we defined hyponatraemia as PNa <136 mmol/l, and severe hyponatraemia as PNa <130 mmol/l, or any level of hyponatraemia associated with symptoms. We also examined hypernatraemia since the arguments against the use of isotonic solutions in children include renal solute loading and the risk of increasing PNa. We defined hypernatraemia as PNa >145 mmol/l.
Data abstraction and study quality
In duplicate and independently, we abstracted data to describe the methodological quality and clinical characteristics of these trials. We contacted authors where necessary for additional data on outcomes of interest. We extracted the following information: study population, sample size, intervention, duration, and type of exposure and outcomes. The methodological quality of included studies was assessed using predefined criteria (Appendices 2 and 3; see http://www.archdischild.com/supplemental).
Data analysis
Cohen's Kappa statistic was used to calculate agreement between raters. For categorical outcomes, treatment effects were expressed as odds ratios (OR) and 95% confidence intervals (CI). We described treatment effects of continuous outcomes using weighted mean differences (WMD) and 95% CI. We calculated summary risk differences and 95% CI using a random effects model (RevMan Version 4.2). Where statistical pooling was not possible, we described our findings qualitatively.
Results
Study selection
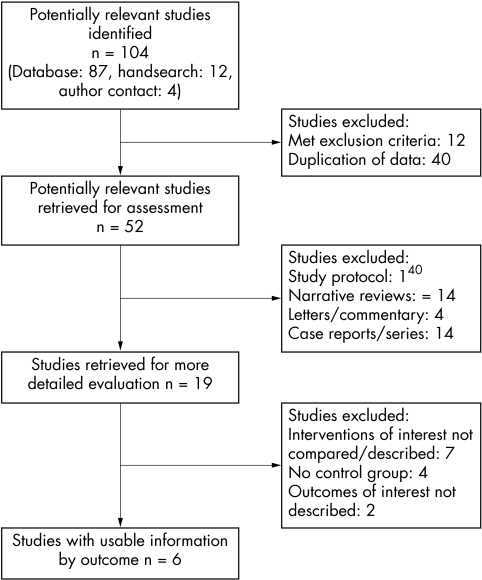
We identified 52 potentially relevant articles from 104 citations (fig 1); 33 did not meet inclusion criteria. Of the 19 studies retrieved for detailed evaluation, seven did not describe or compare the interventions of interest, four did not describe a control group, and two did not report any of the outcomes of interest. Six studies satisfied all criteria (table 1). Cohen's Kappa for inclusion decisions was 0.81 (almost perfect agreement).
Figure 1 Flow diagram of the study selection process for this systematic review.33
Table 1 Characteristics of included studies.
| Brazel (1996)14 | Dagli (1997)16 | Neville (2006)15 | Hoorn (2004)18 | Burrows (1983)17 | Wilkinson (1992)19 | |
|---|---|---|---|---|---|---|
| Participants | ||||||
| n | 12 | 60 | 104 | 148 | 24 | 56 |
| Age (years) | 12.3–18.1 | 1–12 | 6 months–14 years | 7±6 | 6–16 | 2 months–14 years |
| Inclusion criteria | Adolescent females undergoing idiopathic scoliosis repair | ASA 1 patients undergoing elective minor surgery | Gastroenteritis with dehydration | 37 patients with hospital acquired hyponatraemia, 111 isonatraemic historical controls | Previously healthy patients with idiopathic scoliosis undergoing surgical correction | Craniofacial surgery |
| Methodology | RCT, unmasked | Controlled trial | RCT, unmasked | Case control | Cohort study | Retrospective chart review |
| Intervention (all solutions included appropriate dextrose content unless otherwise stated) | Near isotonic solution (LR), n = 5; v hypotonic solutions: (0.3%–0.18% NaCl), n = 7 | Gp 1: LR Gp 2: 1% Dextrose in LR Gp 3: 3.3% Dextrose in 0.3% NaCl | Gp1: 0.45% NaCl Gp 2: 0.9% NaCl | Standard prescription for maintenance IV fluids | Postoperative maintenance fluids: Isotonic (LR), n = 4 Hypotonic (0.25–0.5% NaCl), n = 20 | Isotonic (LR or NS), n = 30 Hypotonic (0.16–0.5% NaCl), n = 26 |
| Outcomes | ||||||
| PNa mmol/l | Greater and more sustained drop in PNa in hypotonic group (p<0.01) | Post‐op PNa in Gp 3 significantly lower (p<0.05). No significant change in Gp 1 and 2 | Mean PNa after 4 hours: Gp 1 134.3 mmol/l (2.1) Gp 2 136.3 mmol/l (3.3) | Cases: PNa dropped from 139±3 to 133±2 mmol/l in 19±10 hours Controls: PNa 140±2 mmol/l | Greater fall in PNa in hypotonic group: 6.2±2.9 mEq/l (p⩽0.05); 3.0±0.8 mEq/l in isotonic group | Median PNa: 130.5 (121–136) in hypotonic Gp; 139 in isotonic group |
| Hyponatraemia (PNa <136) | 1 patient in LR group, 7 in hypotonic group | PNa in hypotonic group (Gp 3): 133.3±4.6 mEq/l (p<0.05) | 21/31 in Gp 1, 2/21 in Gp 2 | All cases by definition | Post‐op PNa: 131±2.8 in hypotonic group; 135±1.9 mmol/l in isotonic group | 20/26 patients in hypotonic group, 2/30 in isotonic group |
| Severe hyponatraemia (PNa <130) | 4 in hypotonic group | Not described | 5/22 in Gp 1 (PNa ⩽130); 0/22 in Gp 2 | Not described | 5 patients in hypotonic group | 11 in hypotonic group |
| Clinical sequelae related to hyponatraemia | Not mentioned | Not described | None described | More nausea and vomiting reported in hyponatraemic group | Increased interstitial pulmonary fluid in hypotonic group (p<0.05) | Seizures: 2/26 in hypotonic group |
| Hypernatraemia (PNa >145) | None | None | None | None | None | None |
CVS, cardiovascular; LR, Lactated Ringers; NS, normal saline; PNa, plasma sodium; pre/post‐op, pre‐ or postoperative; Gp, group; NaCl, sodium chloride; RCT, randomised controlled trial.
Study characteristics
We report the characteristics of the six included studies in table 1. There were two unmasked randomised controlled trials (RCT),14,15 and one non‐randomised controlled trial.16 Three were observational studies.17,18,19Tables 3–5 outline the study quality and methodological characteristics—the overall quality of included studies was often limited; allocation concealment, blinding of patients, clinicians, outcomes assessors, and outcomes were inconsistently or not reported across studies.
Table 2 Characteristics of excluded studies.
| Study | Methods | Participants | Interventions | Primary outcome of study | Reason for exclusion |
|---|---|---|---|---|---|
| Neville (2005)15 | Cohort study | Children with gastroenteritis (n = 52) | Hypotonic IV fluids | PNa, osmolality, ADH, urine electrolytes and osmolality, cortisol, and thyroid hormone | No control group |
| Cupido (2000)25 | Cohort study | Post‐op craniofacial patients (n = 16) | Isotonic fluid | PNa | No control group |
| Halberthal (2001)30 | Retrospective chart review | Hospital acquired severe hyponatraemia within 48 h admission (n = 23). | Hypotonic fluid | Factors contributing to hospital acquired hyponatraemia | No control group |
| Gerick (1996)21 | Case‐control study | 103 cases, 31 age matched controls | IV/PO fluid therapy | ADH and plasma renin activity in cases v controls | Outcomes of interest not described |
| Levine (2001)24 | Cohort study | Craniofacial patients (n = 10) | Isotonic IV fluid | Serum and urine electrolytes | No control group |
| Judd (1990)34 | Case‐control study | Tonsillectomy (n = 13) | Gp 1: perioperative NS IV fluid. GP 2: NPO, no IV fluids | Serum electrolytes, ADH, and plasma renin activity | Only one intervention of interest described |
| Duke (2002)35 | RCT | Children with meningitis | Hypotonic IV fluids v moderate oral fluid restriction | Survival and neurological status | Only one intervention of interest described |
| Cowley (1988)20 | Cohort study | 8 healthy children undergoing scoliosis repair | Type of fluids not described individually | Serum and urine electrolytes, ADH and renin activity | Type of fluids not individually described |
| Arieff (1999)36 | Retrospective chart review | Fatal cases of post‐op hyponatraemia | Not described | Volume of fluid administered | Intervention not described, primarily adult study |
| Wattad (1992)37 | Retrospective chart review | Patients admitted with hyponatraemia | Not described | Aetiology of hyponatraemia | Interventions of interest not described |
| Dunn (1997)38 | Retrospective chart review | Patients with PNa>165 or Na<130 | Not described | Aetiology of hospital acquired PNa derangements | Interventions of interest not described |
| McCormick (1999)39 | Retrospective chart review | Elective paediatric general surgical cases | Hypotonic or isotonic fluids | Not described | Outcomes of interest not described |
| Powell (1990)23 | RCT | Children with meningitis | Fluid restriction v maintenance plus deficit replacement | PNa, plasma AVP levels | Type of fluids not individually described |
ADH, antidiuretic hormone; IV, intravenous; PO, oral.
Table 3 Quality assessment; controlled trials.
| Author | Subjects | Intervention | Outcomes | Follow up | Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Description of subjects | Allocation concealment | Method of randomisation described | Well defined/objective interventions | Care taker/pt blinding | Definition | Blinding | Sufficient (⩾90%) | I TT | Adjustment for confounders | Data provided to confirm results | |
| Brazel | Yes | No | No | Yes | No | Yes | No | Yes | No | No | Yes |
| Dagli | Yes | No | No | Yes | No | Yes | No | Yes | No | No | Yes |
| Neville | Yes | Unclear | Yes | Yes | No | No | No | Yes | No | No | Yes |
Table 4 Quality assessment; observational studies—cohort studies.
| Author | Selection | Comparability | Outcome | |||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of exposed cohort | Selection of non‐exposed cohort | Ascertainment of exposure | Outcome of interest not present at start of study | Assessment | Follow up: outcomes | Follow up: cohorts | ||
| Burrows | * | * | * | * | ||||
| Wilkinson | * | * | * | * | * | |||
Table 5 Quality assessment; observational studies—case‐control studies.
| Author | Selection | Comparability | Exposure | |||||
|---|---|---|---|---|---|---|---|---|
| Case definition | Representativeness | Selection of controls | Definition of Controls | Ascertainment | Method of ascertainment | Non‐response rate | ||
| Hoorn | * | * | * | * | * | * | * | |
Clinical outcomes
Plasma sodium
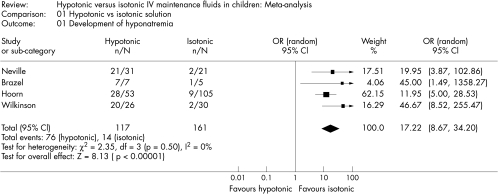
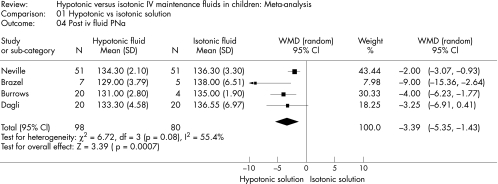
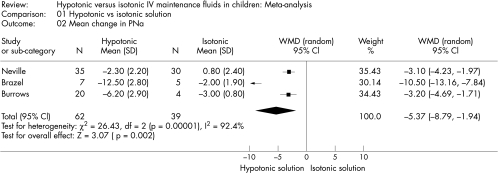
The standard deviations (SD) were not presented for PNa in one of the studies.14 Thus, we calculated a pooled SD to compare the PNa across studies. Hypotonic maintenance solutions significantly increased the risk of developing hyponatraemia (OR 17.22; 95% CI 8.67 to 34.2) (fig 2). Mean PNa in patients following hypotonic solutions was significantly lower (−3.39 mmol/l; 95% CI −5.35 to −1.43), than those who received isotonic solutions (fig 3). The PNa also decreased significantly greater in patients who received hypotonic solutions (−5.37 mmol/l; 95% CI −8.79 to −1.94, fig 4). None of the studies reported the development of hypernatraemia. However, three studies reported a decrease in PNa despite the infusion of isotonic or near‐isotonic IV maintenance fluids (table 1).15,17
Figure 2 Forrest plot summarising the odds ratios and associated 95% confidence intervals for developing hyponatraemia in children receiving hypotonic compared to isotonic IV maintenance fluids.
Figure 3 Comparison of PNa levels following hypotonic versus isotonic or near‐isotonic IV maintenance fluids.
Figure 4 Mean change in PNa following hypotonic versus isotonic IV maintenance fluids.
Morbidity attributed to hyponatraemia
Adverse clinical outcomes were reported in three studies.17,18,19 Wilkinson reported seizures in 2/26 patients receiving hypotonic fluids (OR 6.22; 95% CI 0.29 to 135.8).19 Hoorn reported nausea and vomiting more commonly in patients with hospital acquired hyponatraemia (68%, p = 0.008)18 than isonatraemic controls. The presence of increased pulmonary interstitial fluid on chest x ray was reported by Burrows in 15/20 of patients receiving hypotonic solutions and 2/4 in the near‐isotonic group.20 The clinical significance of this finding was not commented on by the authors. Other outcomes of interest as listed in our objectives were not reported.
Volume of IV fluid administration
Hoorn reported that patients with hospital acquired hyponatraemia did not receive significantly greater total fluid volume than isonatraemic patients, however the calculated electrolyte‐free water intake was three times greater compared to the isonatraemic controls (p < 0.001). The total sodium intake in mmol/kg/h was not significantly different between the two groups.18 The volume of IV fluid infused was not a determinant of the change in PNa at four hours in Neville's study of patients with gastroenteritis.15 Fluid balance and volumes of fluid infused were not specifically presented in the other studies, but described as “same in both groups”.
Subgroups
Four of the included studies were in surgical patients,14,16,17,19 and one study enrolled patients with gastroenteritis.15 Hoorn identified more surgical patients in the hospital acquired hyponatraemia group (16%), than in the isonatraemic controls (5%, p = 0.04).18 All studies examined associations using univariate analyses; none used multivariate analyses to adjust for confounding factors.
Heterogeneity
Given the small number of studies, we chose to include and analyse results from both controlled trials and observational studies. Visual inspection of the Forrest plots indicated study heterogeneity; however formal statistical tests in this instance are underpowered to detect and adjust for clinically important heterogeneity, given the small number of outcomes, patients, and studies. We thus chose to describe the sources of clinical heterogeneity. (1) Patients included in this systematic review were heterogeneous, however the majority of studies were in the surgical population. (2) The degree of exposure to the interventions varied between studies—the timing of PNa measurements occurred after variable degrees and duration of exposure to intervention. (3) The majority of studies were limited in their quality (tables 3–5). Despite apparent heterogeneity in study design, participants, and quality among these studies, the treatment effect nevertheless appears to be remarkably consistent across the studies.
Discussion
Intravenous fluids are used in children to either expand a contracted ECF space or as “maintenance” to replace urine output and insensible losses. In the former instance isotonic or near‐isotonic saline is recommended on the basis that it is the physiologically appropriate solution. In the latter case hypotonic saline solutions are the accepted standard of care. This systematic review reveals that the evidence for the safety of this ubiquitous practice is limited, with only six published studies (only two of which were RCTs) reporting data on a total of 404 patients. The current level of evidence suggests that hypotonic maintenance solutions in children are not benign, but in fact potentially dangerous. The overall treatment effect is remarkable with the odds of developing hyponatraemia following hypotonic solutions being 17.2 times greater than with isotonic fluids. Hence, there are potential risks associated with the use of hypotonic solutions in children, such as cerebral oedema precipitated by an acute fall in serum osmolality.
Hyponatraemia occurs due to a positive balance of electrolyte free water, combined with an impaired ability to excrete hypotonic urine secondary to ADH secretion. A significant correlation between free water intake and decrease in PNa has been demonstrated.20 The primary source of electrolyte free water is the exogenous administration of hypotonic fluid. In contrast to healthy individuals, hospitalised patients have multiple non‐osmotic stimuli for ADH secretion, which prevents them from producing water diuresis even in the presence of a PNa that is lower than 136 mmol/l.12,15,21 In such patients, there will be very little if any excretion of electrolyte free water, because ADH makes the later parts of the distal nephron permeable to water.22 The risk of hyponatraemia in these patients is under‐recognised,14,17,21 and is thus compounded by the administration of hypotonic solutions. However, the administration of isotonic maintenance solutions at least in children with meningitis, has been shown to result in a more rapid return of ADH to normal concentrations, when compared to hypotonic fluids.23 Neville demonstrated that patients admitted with gastroenteritis have obligate urinary sodium losses irrespective of initial PNa.15 The urinary tonicity at presentation of these patients approximates that of normal saline. Therefore infusion of a hypotonic solution which is lower in tonicity than that of urine passed is predictive of a decrease in subsequent PNa.
The concern that isotonic maintenance fluids may cause hypernatraemia is not supported in the studies we reviewed, nor is it reported in adults where the use of isotonic solutions is routine. On the contrary, the risks of hyponatraemia may also extend to patients who receive isotonic fluid.14,15,16,21,24 This can be explained at least in part by the excretion of relatively hypertonic urine as demonstrated by Neville and others.15,24,25 Steele observed that the expansion of the ECF with Ringers Lactate in the perioperative period results in the production of a hypertonic urine resulting in “desalination”.26 However, hypernatraemia can occur during the administration of isotonic saline if a hypotonic urine is produced, leading to a positive sodium balance.
What is already known on this topic
The current standard of prescribing maintenance IV fluids is based on historical evidence
The safety of this practice is yet to be tested in well conducted clinical trials
The traditional guidelines for fluids in children, published 50 years ago, and more recently reiterated,27,28 were derived from estimates of insensible water losses, and electrolyte requirements for normal growth.1 These calculations have since been criticised, and may lead to an overestimation of hypotonic fluid requirement in sick children.6,29 It has been demonstrated that it is not simply Na+ intake, but moreover its ratio to electrolyte free water intake that influences PNa.18 These findings challenge the previous recommendations made by Holliday and Segar, and argue for a maintenance solution and volume which maintains tonicity balance during acute illness, rather than one which merely provides a daily sodium or caloric requirement. We used PNa as a surrogate measure of morbidity related to fluid shifts between intra‐ and extracellular compartments. PNa is a convenient marker as it reflects the ratio between effective osmoles and total body water. As Na+ is the principal extracellular cation and therefore the main determinant of ECF volume, it regulates water movement across cell membranes and explains the development of intracellular oedema that occurs in the presence of hyponatraemia. The expansion of intracellular fluid volume is of major importance in the central nervous system as the brain is confined in a rigid bony cage and has only limited ability to expand. Thus brain cell swelling is very likely to increase intracranial pressure and predispose to brain herniation. Children are at greater risk of this sequela because their brains have a larger intracellular fluid volume per total skull volume.30 Certainly among children who develop symptomatic hyponatraemia, the incidence of permanent brain damage is substantially higher than in adults.31
The results of this systematic review validate the growing concerns expressed in reports which question the safety of our current practice.13,32 The strengths of this report include a comprehensive search strategy, explicit selection criteria for relevant primary studies, reliability assessment of study screening and study quality, validity assessment of primary studies, statistical pooling of effect sizes, focus on adverse events, and reporting according to QUOROM guidelines.33 The weaknesses are that most studies reviewed were heterogeneous in design, small, and of variable quality, did not allow for confounding factors, and focused on a limited paediatric population. Therefore we cannot state with certainty that the principles are applicable to all children prescribed IV maintenance fluids. On the other hand, we can state that, based on published case reports of deaths and neurological injury from acute hyponatraemia that the administration of hypotonic solutions to children with a PNa <138 mmol/l is potentially hazardous, given the fact that ADH is likely to be acting.
Conclusions
The current practice of prescribing IV maintenance fluids in children is not based on clinical experimental evidence using patient‐important outcomes, and does not provide optimal fluid and electrolyte homoeostasis in hospitalised children. There is evidence that, at least in some paediatric patients, hypotonic solutions exacerbate the risks of hyponatraemia, while isotonic solutions may be protective. Our current responsibility however, is to refrain from adopting a “new standard of care”, until rigorous clinical trials comparing the safety and effectiveness of different IV fluid regimens in children have been completed.
What this study adds
This is the first systematic review which examines the evidence for standard IV maintenance solutions in children
This review provides evidence that, at least in some paediatric patients, hypotonic solutions exacerbate the risks of hyponatraemia, while isotonic solutions may be protective
Supplementary Material
Acknowledgements
The authors wish to thank Dr Deborah Cook (Professor, Departments of Medicine and Clinical Epidemiology and Biostatistics, McMaster University), for her assistance in the preparation of this manuscript.
Abbreviations
CI - confidence interval
ECF - extracellular fluid
IV - intravenous
PNa - plasma sodium
RCT - randomised controlled trial
WMD - weighted mean difference
Footnotes
Competing interests: none declared
References
- 1.Holliday M A, Segar M E. The maintenance need for water in parenteral fluid therapy. Pediatrics 195719823–832. [PubMed] [Google Scholar]
- 2.Wallace W M. Quantitative requirements of infant and child for water and electrolytes under varying conditions. Am J Clin Pathol 1953231133–1141. [DOI] [PubMed] [Google Scholar]
- 3.Darrow D C, Pratt E L. Fluid therapy, relation to tissue composition and expenditure of water and electrolytes. JAMA 1950143432–439. [DOI] [PubMed] [Google Scholar]
- 4.Siegel N J. Fluids, electrolytes and acid‐base. In: Rudolph's pediatrics. 21st edn. New York: McGraw Hill, 2003
- 5.Greenbaum L A. Pathophysiology of body fluids and fluid therapy. In: Nelson's textbook of pediatrics. 17th edn. Philadelphia: W.B. Saunders, 2004
- 6.Shafiee M A S, Bohn D, Hoorn E J.et al How to select optimal maintenance intravenous fluid therapy. QJM 200396601–610. [DOI] [PubMed] [Google Scholar]
- 7.Arieff A I, Ayus J C, Fraser C L. Hyponatraemia and death or permanent brain damage in healthy children. BMJ 19923041218–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armour A. Dilutional hyponatremia: a cause of massive fatal intra‐operative cerebral edema in a child undergoing renal transplantation. J Clin Pathol 199750444–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes P D, Nichols D, Mutton P M. Postoperative hyponatremic encephalopathy: water intoxication. Aust N Z J Surg 199868165–168. [DOI] [PubMed] [Google Scholar]
- 10.Peeters A, Claes J, Saldien V. Lethal complications after tonsillectomy. Acta oto‐rhino‐laryngologica Belg 200155207–213. [PubMed] [Google Scholar]
- 11.Paut O, Remond C, Lagier P. Severe hyponatremic encephalopathy after pediatric surgery: report of seven cases and recommendations for management and precention. Ann Fr Anesth Reanim 200019467–473. [DOI] [PubMed] [Google Scholar]
- 12.Duke T, Molyneux E M. Intravenous fluids for seriously ill children: time to reconsider. Lancet 20033621320–1323. [DOI] [PubMed] [Google Scholar]
- 13.Taylor D, Durward A. Pouring salt on troubled waters. Arch Dis Child 200489411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brazel P, McPhee I P. Inappropriate secretion of antidiuretic hormone in postoperative scoliosis patients: the role of fluid management. Spine 199621727. [DOI] [PubMed] [Google Scholar]
- 15.Neville K, Verge C, Rosenberg A.et al Isotonic is better than hypotonic saline for intravenous rehydration of children with gastroenteritis: a prospective randomised study. Arch Dis Child 200691226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dagli G, Orhan M E, Kurt E. The effects of different hydration fluids used in pediatric anaesthesia on blood glucose, electrolytes, and cardiovascular stability. GATA Bulteni 199739146–152. [Google Scholar]
- 17.Burrows F, Shutack J G, Crone R. Inappropriate secretion of antidiuretic hormone in a post surgical pediatric population. Intensive Care Med 198311527–531. [DOI] [PubMed] [Google Scholar]
- 18.Hoorn E J, Geary D, Robb M.et al Acute hyponatremia related to intravenous fluid administration in hospitalized children: an observational study. Pediatrics 20041131279–1284. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson E, Rieff J, Rekate H.et al Fluid, blood, and blood product management in the craniofacial patient. Pediatr Neurosurg 19921848–52. [DOI] [PubMed] [Google Scholar]
- 20.Cowley D M, Pabari M, Sinton T J.et al Pathogenesis of postoperative hyponatremia following correction of scoliosis in children. Aust N Z J Surg 198858485–489. [DOI] [PubMed] [Google Scholar]
- 21.Gerigk M, Gnehm H, Rascher W. Arginine vasopressin and renin in acutely ill children: implication for fluid therapy. Acta Paediatr 199685550–553. [DOI] [PubMed] [Google Scholar]
- 22.Roberston G L.Vasopressin. Philadelphia, PA: Lippincott Williams and Wilkins, 2000
- 23.Powell K R, Sugarmann L I, Eskanazi A E. Normalisation of plasma arginine vasopressin concentrations when children with meningitis are given maintenance plus replacement fluid therapy. J Pediatr 1990117515–522. [DOI] [PubMed] [Google Scholar]
- 24.Levine J P, Stelnicki E, Weiner H L.et al Hyponatremia in the postoperative craniofacial pediatric patient population: a connection to cerebral salt wasting syndrome and management of the disorder. Plastic & Reconstructive Surgery 20011081501–1508. [DOI] [PubMed] [Google Scholar]
- 25.Cupido C, Dalle‐Mulle L, Halperin M L.et alPostoperative desalination in paediatric patients. Toronto: Dept of Critical Care Medicine, The Hospital for Sick Children and Dept of Nephrology, St Michael's Hospital, 2000
- 26.Steele A, Gowrishankar M, Abrahamson S.et al Postoperative hyponatremia despite near‐isotonic saline infusion: a phenomenon of desalination. Ann Intern Med 199712620–25. [DOI] [PubMed] [Google Scholar]
- 27.Chesney C R. The maintenance need for water in parenteral fluid therapy. Pediatrics 1998102(1 Pt 2)399–400. [DOI] [PubMed] [Google Scholar]
- 28.Holliday M A, Segar M E. Reducing errors in fluid therapy management. Pediatrics 2003111424–425. [PubMed] [Google Scholar]
- 29.Hatherill M. Rubbing salt in the wound. Arch Dis Child 200489414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halberthal M, Halperin M L, Bohn D. Lesson of the week: Acute hyponatraemia in children admitted to hospital: retrospective analysis of factors contributing to its development and resolution. BMJ 2001322780–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung H M, Kluge R, Schrier R W.et al Postoperative hyponatremia: a prospective study. Arch Intern Med 1986146333–336. [PubMed] [Google Scholar]
- 32.Moritz M L, Ayus J C. Prevention of hospital‐acquired hyponatremia: a case for using isotonic saline. Pediatrics 2003111227–230. [DOI] [PubMed] [Google Scholar]
- 33.Moher D, Cook D J, Eastwood S.et al Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 19993541896–1900. [DOI] [PubMed] [Google Scholar]
- 34.Judd B A, Haycock G B, Dalton R N.et al Antidiuretic hormone following surgery in children. Acta Paediatr Scand 199079461–466. [DOI] [PubMed] [Google Scholar]
- 35.Duke T, Mokela D, Frank D.et al Management of meningitis in children with oral fluid restriction or intravenous fluid at maintenance volumes: a randomised trial. Ann Trop Paediatr 200222145–157. [DOI] [PubMed] [Google Scholar]
- 36.Arieff A I. Fatal postoperative pulmonary edema: pathogenesis and literature review. Chest 19991151371–1377. [DOI] [PubMed] [Google Scholar]
- 37.Wattad A, Chiang M L, Hill L L. Hyponatremia in hospitalized children. Clin Pediatr 199231153–157. [DOI] [PubMed] [Google Scholar]
- 38.Dunn K, Butt W. Extreme sodium derangement in a paediatric inpatient population. J Paediatr Child Health 19973326–30. [DOI] [PubMed] [Google Scholar]
- 39.McCormick A, Gande R, Lewis I. Postoperative hyponatraemic encephalopathy following elective surgery in children. Paediatr Anaesth 19999551–552. [DOI] [PubMed] [Google Scholar]
- 40.Duke T, Mathur A, Kukuruzovic R.et al Hypotonic vs isotonic saline solutions for intravenous fluid management of acute infections (review). The Cochrane Database of Systematic Reviews 2006(2) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.