Abstract
Objective
To estimate the prevalence of nasopharyngeal (NP) carriage of pneumococcus (Streptococcus pneumoniae) and describe the antibiotic resistance patterns and serotypes in young children attending group day care in London.
Design and subjects
Cross‐sectional survey of attendees at a sample of registered child day care centres (CDCCs) in a London borough.
Setting
Urban setting with a socially and culturally diverse population.
Methods and outcomes
19 CDCCs (13% of total) participated between March and November 2003. A single NP swab was required from each child, and parents completed a questionnaire about their child's health and attendance at day care. WHO methodology for pneumococcal carriage studies was followed.
Results
30% of parents consented. 234 swabs were collected from children aged 6 months to 5 years. 53% were boys and 81% were white. 120 children (51%, 95% CI 45% to 58%) carried pneumococci in their nasopharynx. None of the isolates were resistant to penicillin (upper CL 3%). 21 isolates were resistant to erythromycin (17.5%, 95% CI 11% to 25.5%). 68 isolates (57%) were serotypes included in the 7‐valent conjugate vaccine. Non‐white children had a lower prevalence of carriage (27% vs 58%).
Conclusion: The prevalence of pneumococcal NP carriage was high. The penicillin resistance rate is lower than in many other countries and may reflect a decrease in community antibiotic prescribing in the UK. Monitoring circulating serotypes is important in the context of recent changes to the vaccination policy. Further study is required to explore the association with ethnicity and risk factors for antibiotic resistance.
Out‐of‐home group childcare is associated with an increased risk of infectious illnesses1,2,3 including invasive pneumococcal disease (IPD).4 Carriage studies in child day care centres (CDCCs) have shown increased risk of pneumococcal nasopharyngeal (NP) colonisation5 leading to concerns that CDCCs may act as foci for the emergence and transmission of antibiotic resistant organisms.6,7
Universal childhood vaccination against pneumococcal infection has been adopted in the US, and some European countries include attendance at day care as one of the risk factors for pneumococcal disease in their targeted vaccination strategies. The UK vaccination policy has recently changed to include universal vaccination.8 Previously only children who fell into certain clinical risk groups were recommended to be vaccinated either with the 7‐valent conjugate pneumococcal vaccine (under 5 years of age) or the 23‐valent polysaccharide vaccine and attendance at group day care was not regarded as a risk factor.
Social changes in recent years have already led to many more children under the age of 5 attending group day care9 and a huge expansion of pre‐school day care provision is underway in England.10
There are few UK data on pneumococcal carriage in the day care setting and this study was set up to address this gap. The aim was to determine the prevalence of pneumococcal carriage and resistance, and to describe the prevalent serotypes.
Methods
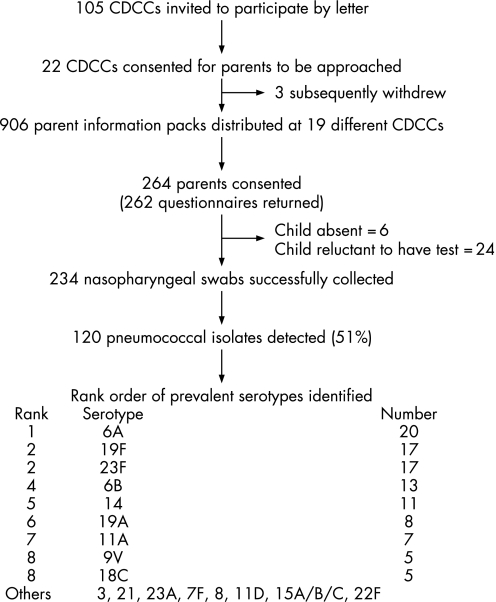
A cross‐sectional study was carried out among infants and children attending a sample of CDCCs across a single inner London borough between March and November 2003 (fig 1), with approval from the Local Research Ethics Committee.
Figure 1 Flow chart of participants and pneumococcal carriage results.
A sample size of 500 children was proposed to allow a 25% carriage rate to be estimated with tight confidence intervals of ±4%.
Day nurseries and nursery schools registered with the local authority were invited to take part. Parent information packs were distributed at participating CDCCs. All children well enough to attend the CDCC were considered eligible for enrolment. Written informed consent from parents was required as well as the assent of the individual child on the day.
A questionnaire was completed and returned by parents. This focused on three areas:
demographic information including ethnic group, household size and parental occupation,
medical and antibiotic history including details of any known medical conditions, hospitalisations and number of ear infections in the last year, as well as antibiotic usage currently or within the preceding 3 months and the indication for and type of antibiotic used, and
pattern of attendance at day care.
Enrolled children had a single NP swab test performed by a single researcher. In accordance with the WHO core methodology for pneumococcal carriage studies, a calcium alginate tipped swab on a flexible aluminium shaft (Bibby Sterilin, UK) was used.11 The swab tip was transported to the laboratory at St George's Hospital NHS Trust in skim milk‐tryptone‐glucose‐glycerol (STGG) transport medium within 4 h.
Aliquots (10 μl) from the STGG media were subcultured onto Columbia blood agar (with 5 μg optochin disc) and streptococcal selective plates, which were read after overnight incubation in 5% CO2 at 37°C. Pneumococcal identification was based on colony morphology and optochin sensitivity, with a bile solubility test where there was uncertainty.
A single pneumococcal colony per specimen was tested for antibiotic sensitivity to oxacillin, erythromycin, tetracycline, cefotaxime and chloramphenicol using the British Society for Antimicrobial Chemotherapy disc diffusion method.12 Serotyping using the Quellung reaction was performed by the Respiratory and Systemic Infection Laboratory of the Health Protection Agency. The original specimen in STGG and a set of pure isolates in 15% glycerol were stored at −70°C.
All microbiological and questionnaire data were anonymised and entered onto a secure database. Statistical analysis was performed using STATA (v 8) statistical software. Categorical variables were explored using frequency tables and cross tabulations. The χ2 test was used to assess the difference between groups and logistic regression to assess the association between the outcome variable (pneumococcal carriage) and each predictor variable, with multiple logistic regression to adjust for confounding, using a backward stepwise approach.
Results
A total of 22 CDCCs initially responded to the invitation, with 19 ultimately taking part (fig 1). These CDCCs were spread across the borough and included a range of facilities from large day nurseries with over 90 children to a small nursery school with just 10 children.
Overall, 30% (range 10–66%) of parents approached consented for their child to take part. Of these 264 children, 30 (11%) did not have a NP swab taken. This was due to the child being absent or unwilling to have the test done on the day. All parents except two returned the questionnaire, although some questionnaires were not fully completed.
The age range of participating children was 6 months to 5 years: 69% were aged between 2 and 4 years and 20% were under 2 years, 53% were boys, 81% of the children were white and 77% of parents belonged to social class 1 or 2 (mainly professional, managerial or technical occupations).
Thirteen children were reported as having a chronic medical condition, with asthma the most common complaint, and 35 children (13%) had been hospitalised in the preceding year. Most admissions were for elective surgery or an infectious disease (measles, chicken pox, herpes simplex infection, gastroenteritis and other non‐specific viral illness or chest infection). No parent reported previous pneumococcal infection. However, two children were admitted with non‐specified pneumonia and one with non‐specified meningitis. In the preceding year 30% of children had experienced one or more ear infections.
Five children had received antibiotics on the study day, in three cases this was part of long‐term prophylaxis for urinary tract infections, and 21% had received a course of antibiotics in the previous 3 months. Only 66% of parents could remember which antibiotic had been taken, and in almost half of cases it was a β‐lactam. Five children had received a macrolide. The most common indication for antibiotic treatment was ear infection.
The majority of children attended CDCC on 4 days a week, with a mean length of stay of 6 h per day. On average children had been attending for 1 year, and age at first attendance ranged from 1 month to 3 years.
Prevalence of pneumococcal carriage
Of 234 NP swabs analysed, 120 grew pneumococcus. The overall carriage rate was 51% (95% CI 44.6% to 57.8%). Pneumococcal carriage of over 40% was seen across each of five age groups, ranging from 43% of 3–4‐year‐old children to 61% of children who were 1–2 years of age.
The number of swabs collected at each CDCC ranged from four to 22. No pneumococcal isolates were found at the smallest centre (10 children of whom four were swabbed), however between one and 14 isolates were identified at the remaining 18 CDCCs, representing a range of carriage of 25–80%.
Antibiotic resistance
None of the 120 pneumococcal isolates tested was resistant to penicillin (upper CL 3% using the exact method).
Of 120 (17.5%) pneumococcal isolates, 21 were resistant to erythromycin, and nine of these were also resistant to tetracycline.
Serotype
Results were available for 119 of 120 pneumococcal isolates. Nineteen different serotypes were identified. The most frequently observed serotype was 6A (20/119) followed by 19F and 23F (17 each) (fig 1). In the 17 centres where more than one child carried pneumococcus, at least two and up to eight different serotypes were identified.
Sixty eight (57%) of the isolates were serotypes that are contained in the 7‐valent pneumococcal conjugate vaccine. Additionally, 20 were serotype 6A which may be cross‐covered by the vaccine, bringing potential coverage to 74%.13,14 A further 11 isolates belonged to vaccine‐associated serogroups, although there is less evidence of cross protection for these.
Sixteen (of 21) antibiotic resistant pneumococci belonged to a serotype contained in the 7‐valent conjugate vaccine, with another one belonging to serotype 6A. Thus, 81% of resistant serotypes seen would be covered by the 7‐valent conjugate vaccine. Of the other four resistant pneumococci, three were serotype 19A and one 15A.
Risk factors
Univariate analysis of each predictor variable in turn found no significant association between pneumococcal carriage and a range of health variables, including number of ear infections, recent history of antibiotic use, chronic medical condition or hospitalisation (table 1).
Table 1 Demography and risk factors by carrier status.
| Characteristic (n) | n | Carriers | Non‐carriers | χ2 Test |
|---|---|---|---|---|
| n (%) | n (%) | p value | ||
| Age (234) | ||||
| 6 months–1 year | 19 | 9 (47) | 10 (53) | 0.2 |
| 1–2 years | 34 | 21 (62) | 13 (38) | |
| 2–3 years | 63 | 35 (56) | 28 (44) | |
| 3–4 years | 93 | 40 (43) | 53 (57) | |
| 4–5 years | 25 | 15 (60) | 10 (40) | |
| Gender (234) | ||||
| Male | 126 | 64 (51) | 62 (49) | 0.87 |
| Female | 108 | 56 (52) | 52 (48) | |
| Ethnicity (232) | ||||
| White | 187 | 108 (58) | 79 (42) | 0.005* |
| Non‐white | 45 | 12 (27) | 33 (73) | |
| Social class (229) | ||||
| 1 and 2 | 179 | 100 (56) | 79 (44) | 0.08 |
| 3 | 24 | 9 (38) | 15 (62) | |
| 4, 5 and other | 26 | 10 (38) | 16 (62) | |
| Household size (229) | ||||
| ⩽3 | 85 | 40 (47) | 45 (53) | 0.59 |
| 4 | 96 | 53 (55) | 43 (45) | |
| 5 | 33 | 19 (58) | 14 (42) | |
| ⩾6 | 15 | 7 (47) | 8 (53) | |
| Ear infections in last | ||||
| 12 months (227) | ||||
| 0 | 161 | 85 (53) | 76 (47) | 0.94 |
| 1 | 36 | 18 (50) | 18 (50) | |
| ⩾2 | 30 | 16 (53) | 14 (47) | |
| Antibiotics today (231) | ||||
| Yes | 5 | 2 (40) | 3 (60) | 0.58 |
| No | 226 | 118 (52) | 108 (48) | |
| Antibiotics in last | ||||
| 3 months (231) | ||||
| Yes | 50 | 22 (44) | 28 (56) | 0.2 |
| No | 181 | 98 (54) | 83 (46) | |
| Hospitalisation in last | ||||
| 12 months (231) | ||||
| Yes | 27 | 15 (56) | 12 (44) | 0.69 |
| No | 204 | 105 (51) | 99 (49) | |
| Chronic medical | ||||
| condition (230) | ||||
| Yes | 12 | 5 (42) | 7 (58) | 0.47 |
| No | 218 | 115 (53) | 103 (47) |
Ethnicity was the only variable which achieved significance on univariate analysis, and this remained significantly associated with pneumococcal carriage when the effect of all other demographic, day care and health variables were taken into consideration. The odds ratio for belonging to a non‐white ethnic group was 0.26 (95% CI 0.12 to 0.54) (adjusted OR 0.27 (95% CI 0.1 to 0.6)).
Discussion
This study is the first published study of pneumococcal carriage and antibiotic resistance in the day care setting in the UK and the first to be conducted in a socially and culturally diverse urban setting.
Half of all children who took part carried the pneumococcus in their nasopharynx. These data are consistent with the prevalence found in other day care carriage studies from northern Europe (The Netherlands (58%), France (54%), Iceland (50%) and Sweden (42%)), however there is significant variation in the proportion of penicillin resistance seen between countries (0% in Netherlands to 56% in France in the same year).15,16,17,18
This study estimated the prevalence of penicillin resistance among isolates to be less than 3%. Although the sample size was small, these data are consistent with most recent UK data from surveillance of invasive disease (2%),19 and from a longitudinal household carriage study (3.7%).20 It is possible that these low rates of penicillin resistance are related to the substantial decrease in antibiotic prescribing (across a range of antibiotics including β‐lactams) for children in England over the last 10 years.21
Analysis of risk factors for pneumococcal carriage did not find any significant association with antibiotic use. Although several other European studies have also failed to find an association,22,23,24 a number of studies have demonstrated a positive association between carriage of penicillin resistant pneumococci and previous antibiotic consumption.25,26,27,28 This study was not powered to assess risk factors for carriage of resistant pneumococci.
Surveillance of IPD in 2000 found 75% of isolates from children under 5 years old were serotypes present in the 7‐valent conjugate vaccine, which increased to 82% if serotype 6A was included.13 In this study of NP carriage among under 5‐year‐old children only 57% of serotypes were those present in the 7‐valent conjugate vaccine with a maximum theoretical protection of 73% (by including serotype 6A, and this did not change when children under 2 years of age were considered separately). The vaccine would however protect against the majority of penicillin‐resistant pneumococcal strains. It is possible that carried isolates are less pathogenic than those identified in invasive disease, but these data are temporally and geographically distinct and comparisons may not be valid. The role of non‐vaccine serotypes and the potential for serotype replacement will become increasingly important now a universal pneumococcal vaccination programme has been introduced, and will need careful monitoring.14
The association between ethnicity and pneumococcal carriage was an unexpected finding and interpretation is difficult as the “non‐white” category was heterogeneous due to small numbers of children belonging to specific ethnic groups. Published data regarding IPD and ethnicity contrast with our findings. In the USA, minority ethnic groups (African Americans, Alaskan natives, American Indians) have been found to be at higher risk of IPD,29,30 despite adjustment for confounding by socio‐economic status. Similar surveillance data for the UK are not available, however a study of 114 cases of pneumococcal meningitis identified by prospective laboratory‐based surveillance in the North East Thames Region demonstrated higher rates of disease in Asians (2.1/100 000) than Caucasians (0.8/100 000) (p = 0.002).31
Pneumococcal infection usually occurs soon after acquisition of a new serotype32 and more prolonged carriage (as may be detected in a cross‐sectional survey) may identify organisms in individuals who are not going to develop invasive disease. Therefore, higher carriage rates among white children would not necessarily lead to higher rates of invasive disease. Conversely, lower pneumococcal carriage among children belonging to non‐white ethnic groups does not imply there will be fewer infections, indeed the opposite may be true, with children at risk of developing invasive disease as and when new strains are acquired. Future carriage studies should record ethnicity and ideally include some form of clinical or case note follow‐up to look for evidence of infection following documented carriage.
What is already known on this topic
Attendance at group day care is associated with an increased risk of pneumococcal infection and carriage of both antibiotic susceptible and resistant pneumococci.
Some European countries consider attendance at day care to be a risk factor for invasive pneumococcal disease and recommend vaccination on this basis.
What this study adds
This is the first study in the UK to estimate the prevalence of pneumococcal carriage and antibiotic resistance in the day care setting.
Half of all infants and children under 5 years of age tested carried pneumococci, with high levels across all age bands.
Penicillin resistance was not found, with an upper confidence limit for resistant strains of 3%.
The main limitation of this study is its small size. Uptake was variable, with poorest uptake associated with local difficulties distributing information about the study. However, the low overall uptake rate (30%) may mean that only more highly educated and/or more health conscious or concerned parents consented, and it is possible that this may have produced a bias towards higher carriage levels. However, resistance levels are consistent with other recent UK data. The initial hypothesis that day nurseries may be foci for resistant organisms was not supported. Although formal cluster analysis was not possible due to small numbers at each CDCC, there was evidence of variability within and between CDCCs, with multiple serotypes identified at individual CDCCs, and resistant pneumococci found at over half of all sites. This study took place in an inner London borough, recruited a range of different CDCCs across the borough, and reflected the ethnic diversity of the area. Although more parents in higher social classes participated, these results may be generalisable to other urban settings in the UK.
Carriage of pneumococcus among healthy pre‐school children attending CDCCs is common and not confined to the youngest age groups. These children represent an important potential pool of infection (the majority of new colonisations in a UK household study were introduced from outside, mainly by children under 5 years of age).20 While the low levels of antibiotic resistance found are reassuring, we would continue to advocate judicious use of antibiotics to prevent increases in levels of resistance in the future. The pneumococcal vaccination policy in the UK has recently been changed to introduce universal childhood vaccination and carriage studies such as this play an important role in providing baseline serotype data for subsequent comparison.
Acknowledgements
Serotyping was preformed at the Respiratory and Systemic Illness Laboratory of the Health Protection Agency.
Abbreviations
CDCC - child day care centre
IPD - invasive pneumococcal disease
NP - nasopharyngeal
STGG - skim milk‐tryptone‐glucose‐glycerol
Footnotes
Financial support for this study was provided by Wyeth Vaccines.
Competing interests: St Georges, University of London has received funding from vaccine manufacturers including Wyeth on behalf of PT Heath for undertaking research. PTH has received funding from Wyeth for attending meetings. No other competing interests declared.
Approval of the Wandsworth Local Research Ethics Committee was sought and obtained in November 2002.
References
- 1.Huskins W C. Transmission and control of infections in out‐of‐home child care. Pediatr Infect Dis J 200019(Suppl)S106–S110. [DOI] [PubMed] [Google Scholar]
- 2.Louhiala P J, Jaakkola N, Ruotsalainen R.et al Form of day care and respiratory infections among Finnish children. Am J Public Health 199585(8)1109–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nafstad P, Hagen J A, Oie L.et al Day care centres and respiratory health. Pediatrics 1999103(4)753–758. [DOI] [PubMed] [Google Scholar]
- 4.Takala A K, Jero J, Kela E.et al Risk factors for primary invasive pneumococcal disease among children in Finland. JAMA 1995273859–864. [PubMed] [Google Scholar]
- 5.Ghaffar F, Friedland I, McCracken G H. Dynamics of nasopharyngeal colonization by Streptococcus pneumoniae. Pediatr Infect Dis J 199918(7)638–646. [DOI] [PubMed] [Google Scholar]
- 6.Allen K D. Multi‐resistant pneumococci in children in day‐care centres. J Infect 199837(1)5–8. [DOI] [PubMed] [Google Scholar]
- 7.de Lencastre H, Tomasz A. From ecological reservoir to disease: the nasopharynx, day‐care centres and drug‐resistant clones of Streptococcus pneumoniae. J Antimicrob Chemother 200250(Suppl S2)75–81. [DOI] [PubMed] [Google Scholar]
- 8.Department of Health Pneumococcal. In: Immunisation against infectious disease ‐ the Green Book. London: DH, 2006:Chapter 25
- 9.Holmes S J, Ardythe L, Morrow A L.et al Child‐care practices: effects of social change on the epidemiology of infectious diseases and antibiotic resistance. Epidemiol Rev 199618(1)10–28. [DOI] [PubMed] [Google Scholar]
- 10.Department for Education and Employment Meeting the childcare challenge. A framework and consultation document. London: HMSO 1998
- 11.O'Brien K L, Nohynek H, the WHO Pneumococcal Vaccine Trials Carriage Working Group Report from a WHO working group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr Infect Dis J 200322e1–11. [DOI] [PubMed] [Google Scholar]
- 12.Andrews J M, for the BSAC Working Party on Susceptibility Testing BSAC standardized disc susceptibility testing method. J Antimicrob Chemother 200178(Suppl 1)43–57. [DOI] [PubMed] [Google Scholar]
- 13.Health Protection Agency Invasive pneumococcal infection, England and Wales: 2000. CDR Wkly 200313(21) [Google Scholar]
- 14.Whitney C G, Farley M M, Hadler J.et al Decline of invasive pneumococcal disease after the introduction of protein‐polysaccharide conjugate vaccine. N Engl J Med 2003348(18)1737–1746. [DOI] [PubMed] [Google Scholar]
- 15.Bogaert D, Engelen M N, Timmers‐Reker A J M.et al Pneumococcal carriage in children in The Netherlands: a molecular epidemiological study. J Clin Microbiol 200139(9)3316–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunais B, Pradier C, Carsenti H.et al Influence of child care on nasopharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae. Pediatr Infect Dis J 200322(7)589–592. [DOI] [PubMed] [Google Scholar]
- 17.Hjaltested E K, Bernatoniene J, Erlendsdottir H.et al Resistance in respiratory tract pathogens and antimicrobial use in Icelandic and Lithuanian children. Scand J Infect Dis 200335(1)21–26. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson P, Laurell M H. Carriage of penicillin‐resistant Streptococcus pneumoniae by children in day‐care centers during an intervention program in Malmo, Sweden. Pediatr Infect Dis J 200120(12)1144–1149. [DOI] [PubMed] [Google Scholar]
- 19.Livermore D M. Minimising antibiotic resistance. Lancet Infect Dis 20055450–459. [DOI] [PubMed] [Google Scholar]
- 20.Hussain M, Melegaro A, Pebody R G.et al A longitudinal household study of Streptococcus pneumoniae nasopharyngeal carriage in a UK setting. Epidemiol Infect 2005133891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharland M, Kendall H, Yeates D.et al Antibiotic prescribing in general practice and hospital admissions for peritonsillar abscess, mastoiditis and rheumatic fever in children: time trend analysis. BMJ 2005331328–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Syrogiannopoulos G A, Grivea I N, Beratis N G.et al Resistance patterns of Streptococcus pneumoniae from carriers attending day‐care centers in southwestern Greece. Clin Infect Dis 199725(2)188–194. [DOI] [PubMed] [Google Scholar]
- 23.Petrosillo N, Pantosti A, Bordi E.et al Prevalence, determinants, and molecular epidemiology of Streptococcus pneumoniae isolates colonizing the nasopharynx of healthy children in Rome. Eur J Clin Microbiol Infect Dis 200221(3)181–188. [DOI] [PubMed] [Google Scholar]
- 24.Marchisio P, Esposito S, Schito G C.et al Nasopharyngeal carriage of Streptococcus pneumoniae in healthy children: implications for the use of heptavalent pneumococcal conjugate vaccine. Emerg Infect Dis 20028(5)479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arason V A, Kristinsson K G, Sigurdsson J A.et al Do antimicrobials increase the carriage rate of penicillin resistant pneumococci in children? Cross sectional prevalence study. BMJ 1996313387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melander E, Molstad S, Persson K.et al Previous antibiotic consumption and other risk factors for carriage of penicillin‐resistant Streptococcus pneumoniae in children. Eur J Clin Microbiol Infect Dis 199817(12)834–838. [DOI] [PubMed] [Google Scholar]
- 27.Yagupsky P, Porat N, Fraser D.et al Acquisition, carriage, and transmission of pneumococci with decreased antibiotic susceptibility in young children attending a day care facility in southern Israel. J Infect Dis 1998177(4)1003–1012. [DOI] [PubMed] [Google Scholar]
- 28.Dagan R, Melamed R, Muallem M.et al Nasopharyngeal colonization in southern Israel with antibiotic‐resistant pneumococci during the first 2 years of life: relation to serotypes likely to be included in pneumococcal conjugate vaccines. J Infect Dis 19961741352–1355. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP).MMWR Morb Mortal Wkly Rep 200048(No.RR‐9)1–35. [PubMed] [Google Scholar]
- 30.Flannery B, Schrag S, Bennett N M.et al Impact of childhood vaccination on racial disparities in invasive Streptococcal pneumoniae infections. JAMA 2004291(18)2197–2220. [DOI] [PubMed] [Google Scholar]
- 31.Urwin G, Yuan M F, Hall L M C.et al Pneumococcal meningitis in North East Thames Region UK: epidemiology and molecular analysis of isolates. Epidemiol Infect 1996117(1)95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray B M, Converse G M, Dillon H C. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis 1980142(6)923–933. [DOI] [PubMed] [Google Scholar]