Abstract
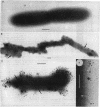
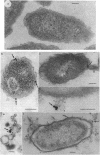
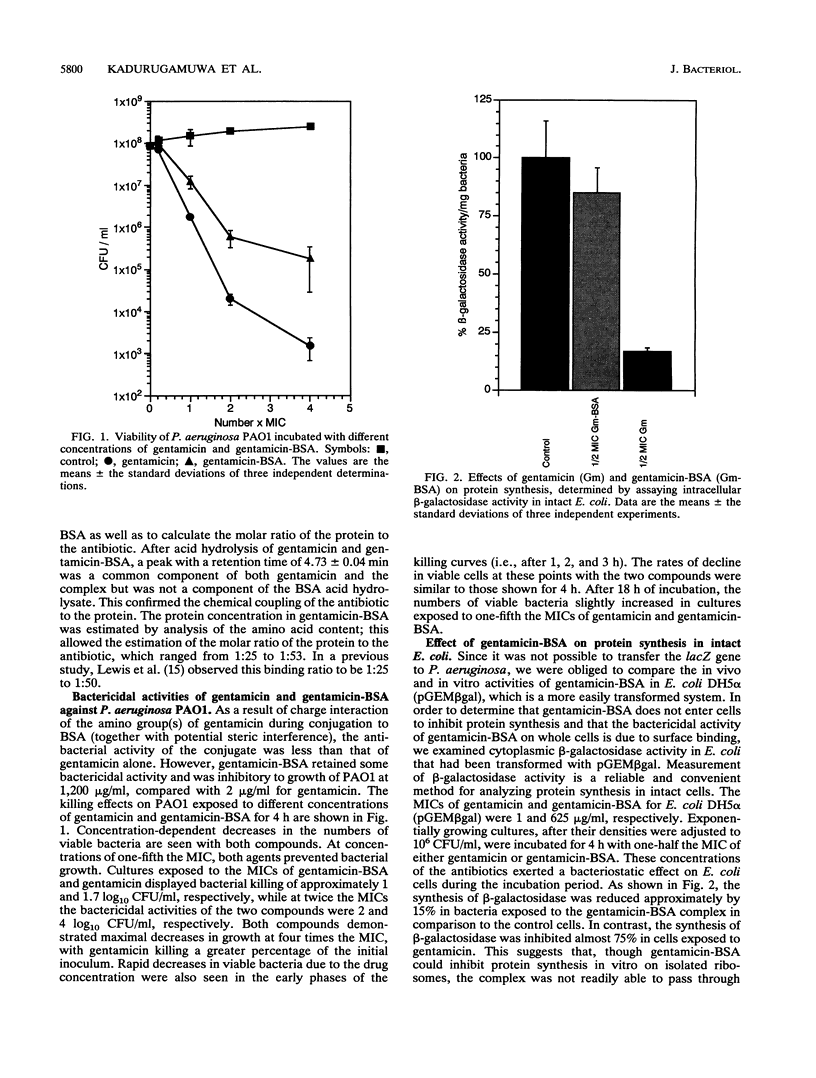
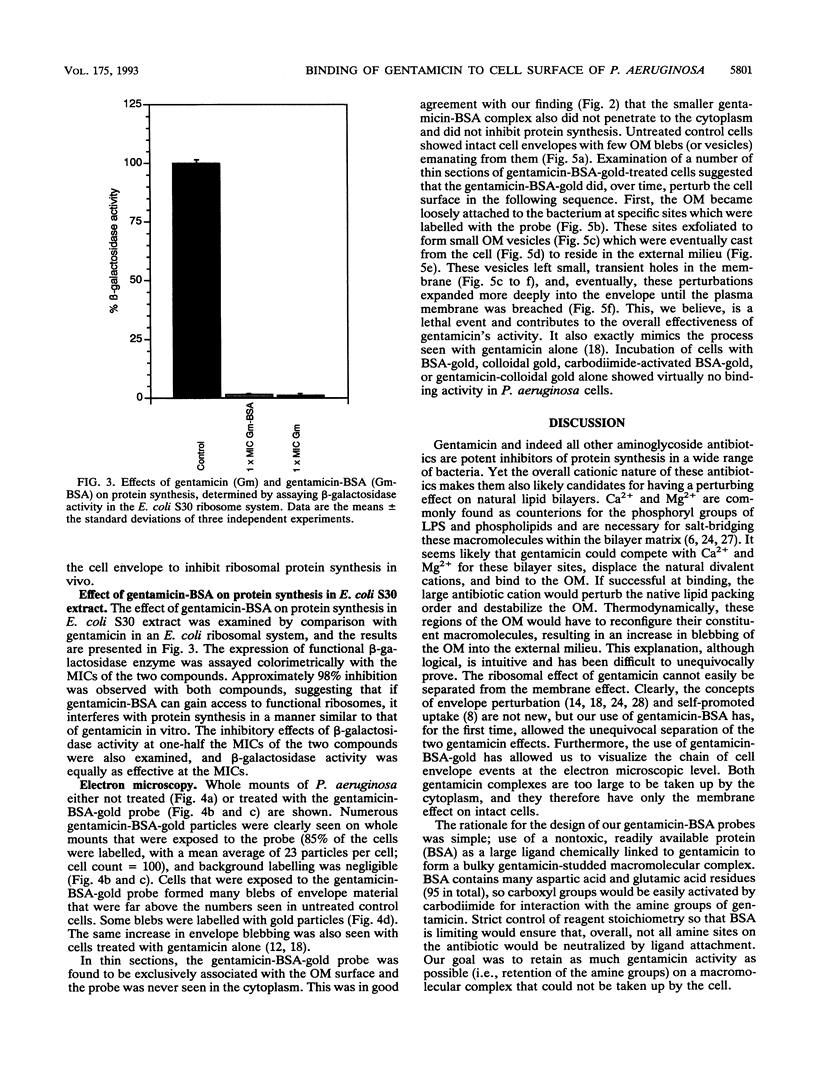
The mode of action of gentamicin has traditionally been considered to be at the 30S ribosomal level. However, the inhibition of bacterial protein synthesis alone appears to be insufficient to entirely explain the bactericidal effects. Bacteriolysis is also mediated through perturbation of the cell surface by gentamicin (J.L. Kadurugamuwa, J.S. Lam, and T.J. Beveridge, Antimicrob. Agents Chemother. 37:715-721, 1993). In order to separate the surface effect from protein synthesis in Pseudomonas aeruginosa PAO1, we chemically conjugated bovine serum albumin (BSA) to gentamicin, making the antibiotic too large to penetrate through the cell envelope to interact with the ribosomes of the cytoplasm. Furthermore, this BSA-gentamicin conjugate was also used to coat colloidal gold particles as a probe for electron microscopy to study the surface effect during antibiotic exposure. High-performance liquid chromatography confirmed the conjugation of the protein to the antibiotic. The conjugated gentamicin and BSA retained bactericidal activity and inhibited protein synthesis on isolated ribosomes in vitro but not on intact cells in vivo because of its exclusion from the cytoplasm. When reacted against the bacteria, numerous gentamicin-BSA-gold particles were clearly seen on the cell surfaces of whole mounts and thin sections of cells, while the cytoplasm was devoid of such particles. Disruption of the cell envelope was also observed since gentamicin-BSA and gentamicin-BSA-gold destabilized the outer membrane, evolved outer membrane blebs and vesicles, and formed holes in the cell surface. The morphological evidence suggests that the initial binding of the antibiotic disrupts the packing order of lipopolysaccharide of the outer membrane, which ultimately forms holes in the cell envelope and can lead to cell lysis. It is apparent that gentamicin has two potentially lethal effects on gram-negative cells, that resulting from inhibition of protein synthesis and that resulting from surface perturbation; the two effects in concert make aminoglycoside drugs particularly effective antibiotics.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryan L. E., Kwan S. Roles of ribosomal binding, membrane potential, and electron transport in bacterial uptake of streptomycin and gentamicin. Antimicrob Agents Chemother. 1983 Jun;23(6):835–845. doi: 10.1128/aac.23.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Van Den Elzen H. M. Effects of membrane-energy mutations and cations on streptomycin and gentamicin accumulation by bacteria: a model for entry of streptomycin and gentamicin in susceptible and resistant bacteria. Antimicrob Agents Chemother. 1977 Aug;12(2):163–177. doi: 10.1128/aac.12.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse H. J., Wöstmann C., Bakker E. P. The bactericidal action of streptomycin: membrane permeabilization caused by the insertion of mistranslated proteins into the cytoplasmic membrane of Escherichia coli and subsequent caging of the antibiotic inside the cells due to degradation of these proteins. J Gen Microbiol. 1992 Mar;138(3):551–561. doi: 10.1099/00221287-138-3-551. [DOI] [PubMed] [Google Scholar]
- Davis B. D. Mechanism of bactericidal action of aminoglycosides. Microbiol Rev. 1987 Sep;51(3):341–350. doi: 10.1128/mr.51.3.341-350.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont C., Clarke A. J. Evidence for N----O acetyl migration as the mechanism for O acetylation of peptidoglycan in Proteus mirabilis. J Bacteriol. 1991 Jul;173(14):4318–4324. doi: 10.1128/jb.173.14.4318-4324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris F. G., Beveridge T. J. Site specificity of metallic ion binding in Escherichia coli K-12 lipopolysaccharide. Can J Microbiol. 1986 Jan;32(1):52–55. doi: 10.1139/m86-010. [DOI] [PubMed] [Google Scholar]
- Hancock R. E. Aminoglycoside uptake and mode of action--with special reference to streptomycin and gentamicin. I. Antagonists and mutants. J Antimicrob Chemother. 1981 Oct;8(4):249–276. doi: 10.1093/jac/8.4.249. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Bell A. Antibiotic uptake into gram-negative bacteria. Eur J Clin Microbiol Infect Dis. 1988 Dec;7(6):713–720. doi: 10.1007/BF01975036. [DOI] [PubMed] [Google Scholar]
- Jackson G. G., Lolans V. T., Daikos G. L. The inductive role of ionic binding in the bactericidal and postexposure effects of aminoglycoside antibiotics with implications for dosing. J Infect Dis. 1990 Aug;162(2):408–413. doi: 10.1093/infdis/162.2.408. [DOI] [PubMed] [Google Scholar]
- Josepovitz C., Pastoriza-Munoz E., Timmerman D., Scott M., Feldman S., Kaloyanides G. J. Inhibition of gentamicin uptake in rat renal cortex in vivo by aminoglycosides and organic polycations. J Pharmacol Exp Ther. 1982 Nov;223(2):314–321. [PubMed] [Google Scholar]
- Kadurugamuwa J. L., Lam J. S., Beveridge T. J. Interaction of gentamicin with the A band and B band lipopolysaccharides of Pseudomonas aeruginosa and its possible lethal effect. Antimicrob Agents Chemother. 1993 Apr;37(4):715–721. doi: 10.1128/aac.37.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. E., Nelson J. C., Elder H. A. Radioimmunoassay of an antibiotic: gentamicin. Nat New Biol. 1972 Oct 18;239(94):214–216. doi: 10.1038/newbio239214a0. [DOI] [PubMed] [Google Scholar]
- Loh B., Grant C., Hancock R. E. Use of the fluorescent probe 1-N-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Oct;26(4):546–551. doi: 10.1128/aac.26.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N. L., Beveridge T. J. Gentamicin interaction with Pseudomonas aeruginosa cell envelope. Antimicrob Agents Chemother. 1986 Jun;29(6):1079–1087. doi: 10.1128/aac.29.6.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga K., Yamaki H., Nishimura T., Tanaka N. Inhibition of DNA replication initiation by aminoglycoside antibiotics. Antimicrob Agents Chemother. 1986 Sep;30(3):468–474. doi: 10.1128/aac.30.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka H., Tachibana M., Amagai T., Suganuma A. Aminoglycoside binding sites in Escherichia coli as revealed by neomycin-gold labeling. J Histochem Cytochem. 1986 Jul;34(7):909–912. doi: 10.1177/34.7.3519754. [DOI] [PubMed] [Google Scholar]
- Nichols W. W. On the mechanism of translocation of dihydrostreptomycin across the bacterial cytoplasmic membrane. Biochim Biophys Acta. 1987;895(1):11–23. doi: 10.1016/s0304-4173(87)80014-9. [DOI] [PubMed] [Google Scholar]
- Peterson A. A., Hancock R. E., McGroarty E. J. Binding of polycationic antibiotics and polyamines to lipopolysaccharides of Pseudomonas aeruginosa. J Bacteriol. 1985 Dec;164(3):1256–1261. doi: 10.1128/jb.164.3.1256-1261.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Taber H. W., Mueller J. P., Miller P. F., Arrow A. S. Bacterial uptake of aminoglycoside antibiotics. Microbiol Rev. 1987 Dec;51(4):439–457. doi: 10.1128/mr.51.4.439-457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992 Sep;56(3):395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S. G., Beveridge T. J. Amikacin disrupts the cell envelope of Pseudomonas aeruginosa ATCC 9027. Can J Microbiol. 1988 Jan;34(1):12–18. doi: 10.1139/m88-003. [DOI] [PubMed] [Google Scholar]