Abstract
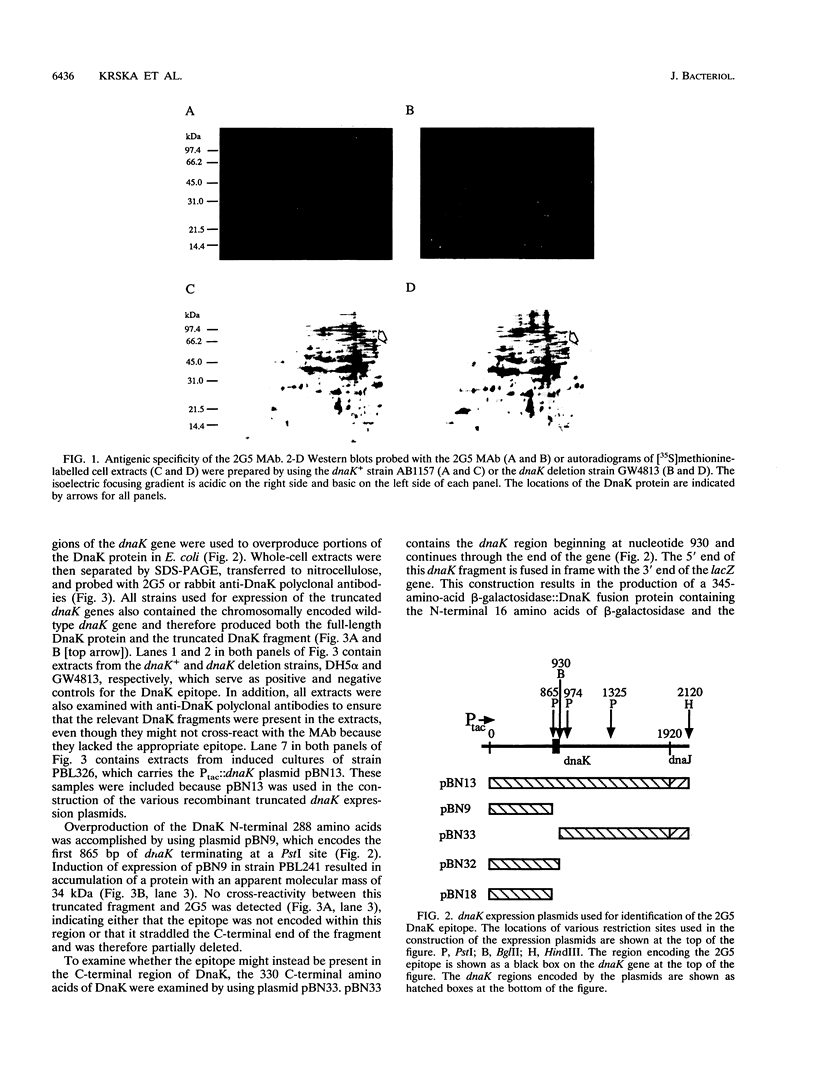
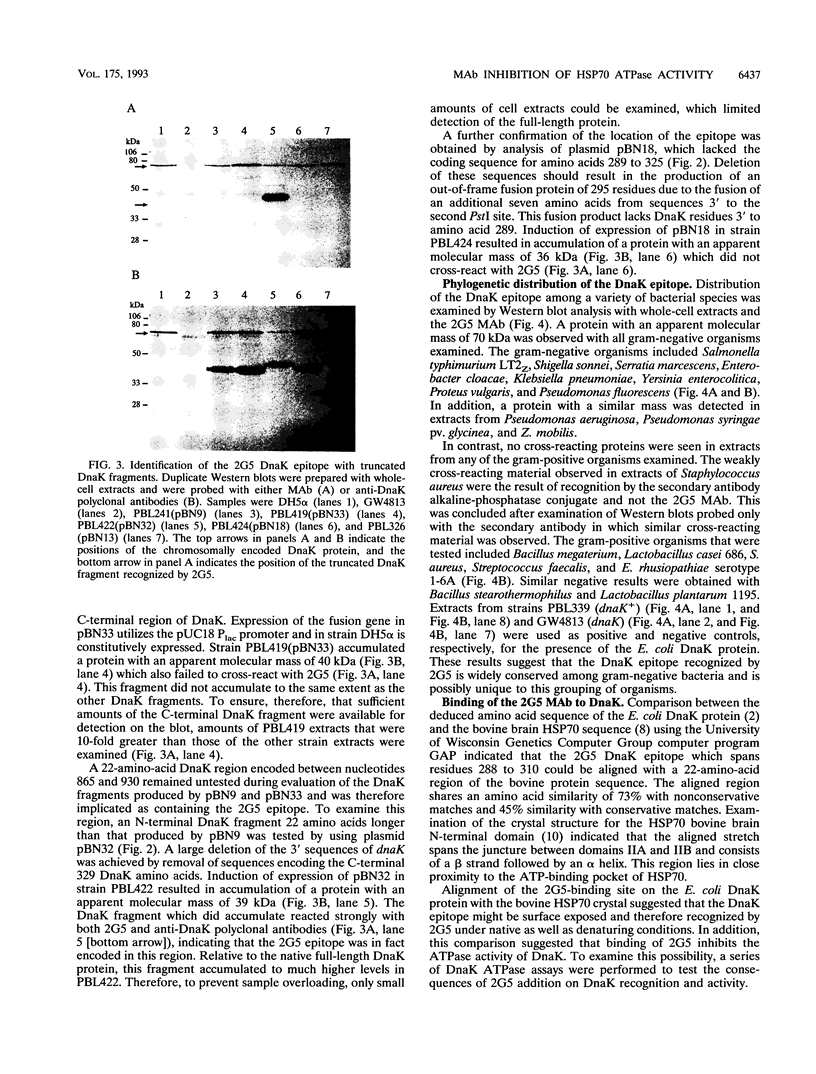
The isolation and characterization of a monoclonal antibody (MAb 2G5) specific for the bacterial DnaK (HSP70) protein is described. The 2G5 MAb was initially selected because of its ability to bind to DnaK under denaturing conditions. Isotype analyses indicated that 2G5 was an immunoglobulin G2a. Dose-response Western blot (immunoblot) experiments with purified but unconcentrated 2G5 permitted detection of 10 ng of pure DnaK protein. The DnaK epitope was determined by Western blot analysis of a series of truncated DnaK fragments overproduced in Escherichia coli using 5' and 3' dnaK-deleted expression plasmids. The epitope mapped to a 22-amino-acid region spanning DnaK residues 288 and 310. Phylogenetic distribution of the epitope was examined by Western blot analysis of a wide variety of bacterial species and indicated that the epitope was uniquely present in gram-negative organisms. The proximity of the epitope to the presumed DnaK ATP-binding pocket suggested that MAb binding might inhibit DnaK ATPase activity. In vitro analysis supported this prediction and demonstrated that MAb-mediated inhibition of ATPase activity was antibody specific and occurred at stoichiometric molar ratios of MAb to DnaK. Possible mechanisms to explain the ability of the 2G5 MAb to inhibit DnaK activity are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balodimos I. A., Rapaport E., Kashket E. R. Protein phosphorylation in response to stress in Clostridium acetobutylicum. Appl Environ Microbiol. 1990 Jul;56(7):2170–2173. doi: 10.1128/aem.56.7.2170-2173.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., Craig E. A. Major heat shock gene of Drosophila and the Escherichia coli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci U S A. 1984 Feb;81(3):848–852. doi: 10.1073/pnas.81.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum P. H., Jovanovich S. B., McCann M. P., Schultz J. E., Lesley S. A., Burgess R. R., Matin A. Cloning and in vivo and in vitro regulation of cyclic AMP-dependent carbon starvation genes from Escherichia coli. J Bacteriol. 1990 Jul;172(7):3813–3820. doi: 10.1128/jb.172.7.3813-3820.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum P., Ory J., Bauernfeind J., Krska J. Physiological consequences of DnaK and DnaJ overproduction in Escherichia coli. J Bacteriol. 1992 Nov;174(22):7436–7444. doi: 10.1128/jb.174.22.7436-7444.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum P., Velligan M., Lin N., Matin A. DnaK-mediated alterations in human growth hormone protein inclusion bodies. Biotechnology (N Y) 1992 Mar;10(3):301–304. doi: 10.1038/nbt0392-301. [DOI] [PubMed] [Google Scholar]
- Cegielska A., Georgopoulos C. Functional domains of the Escherichia coli dnaK heat shock protein as revealed by mutational analysis. J Biol Chem. 1989 Dec 15;264(35):21122–21130. [PubMed] [Google Scholar]
- Chappell T. G., Konforti B. B., Schmid S. L., Rothman J. E. The ATPase core of a clathrin uncoating protein. J Biol Chem. 1987 Jan 15;262(2):746–751. [PubMed] [Google Scholar]
- DeLuca-Flaherty C., McKay D. B. Nucleotide sequence of the cDNA of a bovine 70 kilodalton heat shock cognate protein. Nucleic Acids Res. 1990 Sep 25;18(18):5569–5569. doi: 10.1093/nar/18.18.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., Nickels R. L., McIntosh L. Monoclonal antibodies to the alternative oxidase of higher plant mitochondria. Plant Physiol. 1989 Apr;89(4):1311–1317. doi: 10.1104/pp.89.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty K. M., DeLuca-Flaherty C., McKay D. B. Three-dimensional structure of the ATPase fragment of a 70K heat-shock cognate protein. Nature. 1990 Aug 16;346(6285):623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- Flaherty K. M., McKay D. B., Kabsch W., Holmes K. C. Similarity of the three-dimensional structures of actin and the ATPase fragment of a 70-kDa heat shock cognate protein. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5041–5045. doi: 10.1073/pnas.88.11.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Singh B. Cloning of the HSP70 gene from Halobacterium marismortui: relatedness of archaebacterial HSP70 to its eubacterial homologs and a model for the evolution of the HSP70 gene. J Bacteriol. 1992 Jul;174(14):4594–4605. doi: 10.1128/jb.174.14.4594-4605.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovich S. B., Lesley S. A., Burgess R. R. In vitro use of monoclonal antibodies in Escherichia coli S-30 extracts to determine the RNA polymerase sigma subunit required by a promoter. J Biol Chem. 1989 Mar 5;264(7):3794–3798. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCarty J. S., Walker G. C. DnaK as a thermometer: threonine-199 is site of autophosphorylation and is critical for ATPase activity. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9513–9517. doi: 10.1073/pnas.88.21.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge J., King J., Krska J., Rockabrand D., Blum P. Cloning, heterologous expression, and characterization of the Erysipelothrix rhusiopathiae DnaK protein. Infect Immun. 1993 Feb;61(2):411–417. doi: 10.1128/iai.61.2.411-417.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippmann F., Taylor W. R., Rothbard J. B., Green N. M. A hypothetical model for the peptide binding domain of hsp70 based on the peptide binding domain of HLA. EMBO J. 1991 May;10(5):1053–1059. doi: 10.1002/j.1460-2075.1991.tb08044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzstein M., Völker U., Dedio J., Löbau S., Zuber U., Schiesswohl M., Herget C., Hecker M., Schumann W. Cloning, sequencing, and molecular analysis of the dnaK locus from Bacillus subtilis. J Bacteriol. 1992 May;174(10):3300–3310. doi: 10.1128/jb.174.10.3300-3310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild J., Kamath-Loeb A., Ziegelhoffer E., Lonetto M., Kawasaki Y., Gross C. A. Partial loss of function mutations in DnaK, the Escherichia coli homologue of the 70-kDa heat shock proteins, affect highly conserved amino acids implicated in ATP binding and hydrolysis. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7139–7143. doi: 10.1073/pnas.89.15.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylicz M., LeBowitz J. H., McMacken R., Georgopoulos C. The dnaK protein of Escherichia coli possesses an ATPase and autophosphorylating activity and is essential in an in vitro DNA replication system. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6431–6435. doi: 10.1073/pnas.80.21.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]