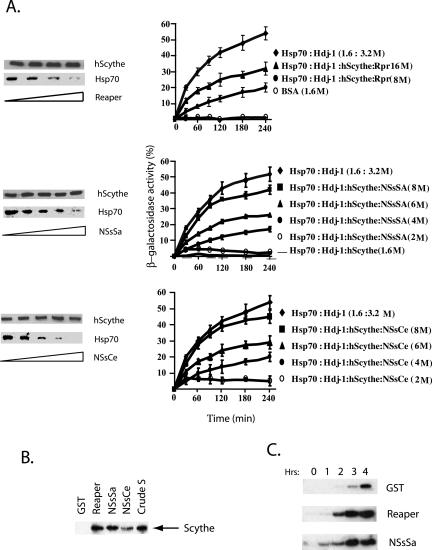
Figure 5.
NSs bind to Scythe and modulate its function. (a) His-hScythe (2 μm) was incubated with Hsp70 (2 μm) and the complex was allowed to form. After complex formation, increasing concentrations of recombinant Reaper (2, 5, 8, and 10 μM in lanes 1–4, respectively), NSsCe or NSsSa (2, 4, 6, 8, and 10 μM in lanes 1–5, respectively) were added. Bound proteins were precipitated with Ni+-agarose beads, washed, resolved by SDS-PAGE, and analyzed by immunoblotting using an Hsp70 mAb 5a5 or an anti-His antibody for hScythe detection (left panel). Hsp 70–dependent refolding of β-galactosidase was measured in the presence of full-length human Scythe and increasing concentrations of recombinant Reaper, NSsSa, or NSsCe (right-panel). (b) Recombinant GST, GST-Reaper, GST-NSsSA, or GST-NSsCe proteins attached to glutathione Sepharose beads were incubated in Xenopus egg extracts, retrieved by centrifugation, washed, and analyzed for the presence of bound Scythe by immunoblotting. (c) Anti-Scythe immunoprecipitates formed by incubation of protein A-linked anti-Scythe antibodies with Xenopus egg extracts were washed and then incubated with recombinant GST, Reaper, or NSsSa protein. The released activity was then incubated with purified isolated mitochondria. After removal of the mitochondria by filtration, released cytochrome c was detected by immunoblotting with anticytochrome c antibody.