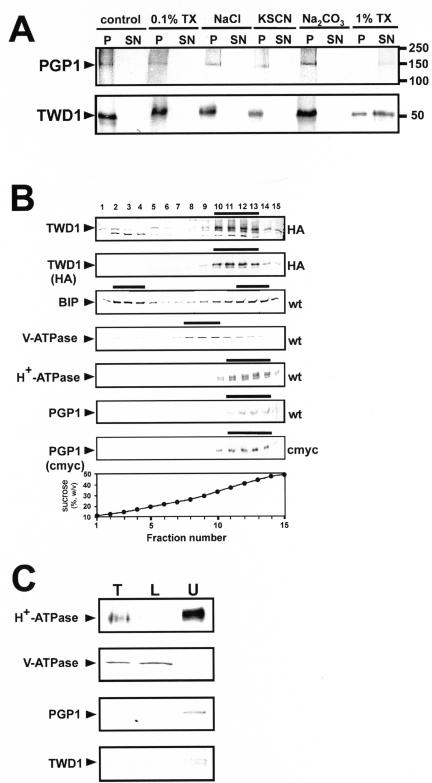
Figure 4.
TWD1 is a plasma membrane-anchored protein. (A) Microsomal fractions expressing an HA-epitope–tagged version of TWD1 or a c-Myc epitope–tagged version of AtPGP1 were treated with 0.1% TX-100, 2 M NaCl, 1 M KSCN, 200 mM Na2CO3, or 1% TX-100. Membranes were pelleted, and supernatants were precipitated with TCA and subjected to PAGE. TWD1 and AtPGP1 were detected using anti-HA and antic-Myc antibodies. (B) Microsomes of wild-type (wt) and transgenic Arabidopsis plants ectopically expressing c-Myc- or HA-epitope–tagged AtPGP1 (c-Myc) and TWD1 (HA), respectively, were subjected to linear sucrose density gradient fractionation. Fractions were immunoprobed against given marker enzymes as described in Geisler et al. (2000). Transgenic plant material was probed additionally against antic-Myc (c-Myc) and anti-HA (HA), respectively. Immunopositive peak fractions are highlighted by bars. (C) Microsomal fractions obtained from aqueous two-phase partitioning of Arabidopsis suspension culture were probed with anti-TWD1 and anti-AtPGP1 antisera. Efficient partitioning of total microsomes (T, 10 μg of protein) to the lower phase (L, 10 μg of protein) or to the upper phase (U, 5 μg of protein) was ascertained by Western blot analysis using antisera against the marker proteins vacuolar V-ATPase and the plasma membrane–bound H+-ATPase.