Abstract
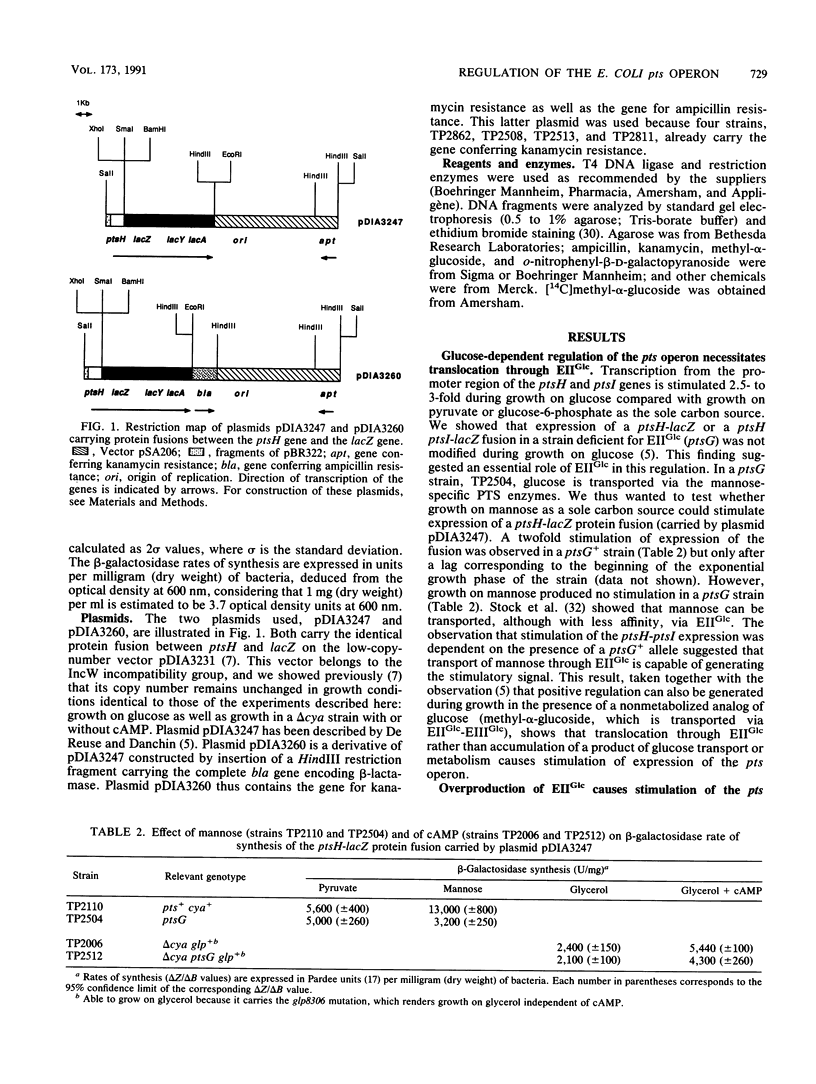
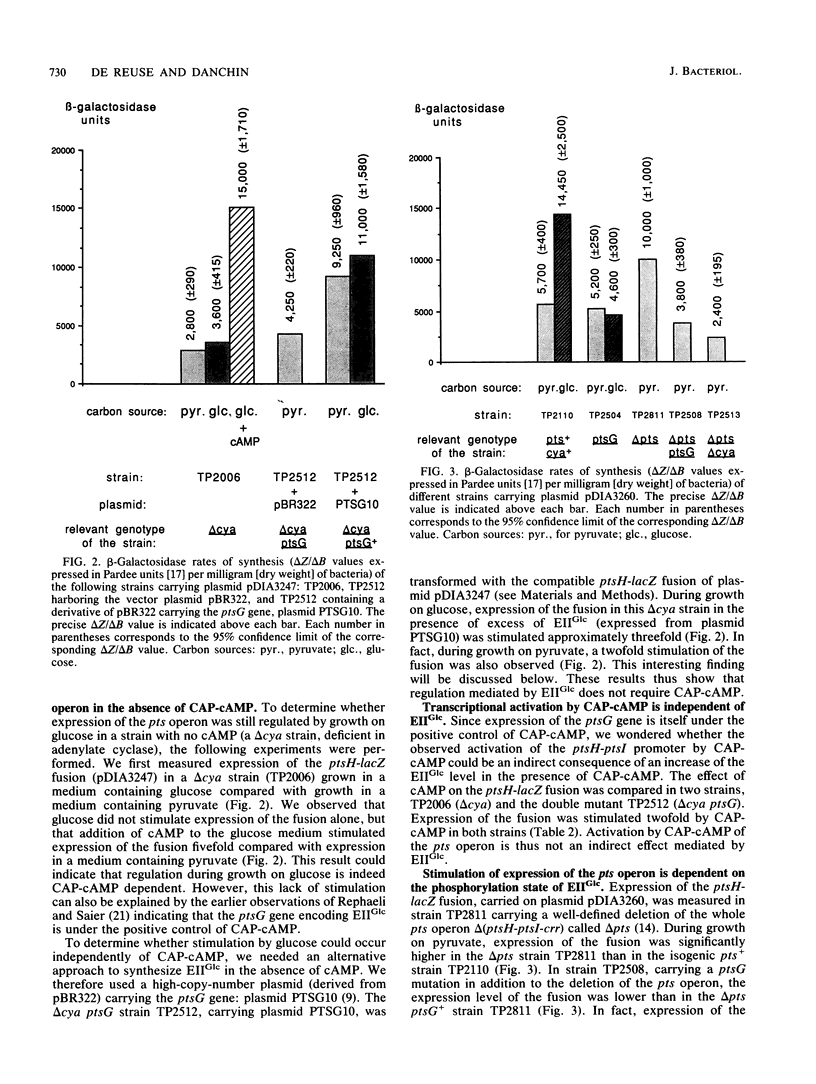
The pts operon of Escherichia coli is composed of the genes ptsH, ptsI, and crr, which code for three proteins of the phosphoenolpyruvate-dependent phosphotransferase system (PTS): the HPr, enzyme I (EI), and EIIIGlc proteins, respectively. These three genes are organized in a complex operon in which the major part of expression of the distal gene, crr, is initiated from a promoter region within ptsI. Expression from the promoter region of the ptsH and ptsI genes has been studied in vivo by using gene fusions with lacZ. Transcription from this promoter region is under the positive control of catabolite activator protein (CAP)-cyclic AMP (cAMP) and is also enhanced during growth in the presence of glucose (a PTS substrate). This report describes a genetic characterization of the mechanism by which growth on glucose causes transcriptional stimulation of the pts operon. This regulation is dependent on transport through the glucose-specific permease of the PTS, EIIGlc. Our results strongly suggest that transcriptional regulation of the pts operon is the consequence of an increase in the level of unphosphorylated EIIGlc which is produced during glucose transport. Furthermore, overproduction of EIIGlc in the absence of transport was found to stimulate expression of the pts operon. We also observed that CAP-cAMP could cause stimulation independently of the EIIGlc and that glucose could activate in the absence of cAMP in a strain overproducing EIIGlc. Our results indicate that glucose acts like an environmental signal through a mechanism of signal transduction. A sequence similarity between the C terminus of EIIGlc and the consensus of transmitter modules of the sensor proteins defined by E. C. Kofoid and J. S. Parkinson (Proc. Natl. Acad. Sci. USA 85:4981-4985, 1988) suggests that EIIGlc might have properties in common with the sensors of the two-component systems.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amster-Choder O., Houman F., Wright A. Protein phosphorylation regulates transcription of the beta-glucoside utilization operon in E. coli. Cell. 1989 Sep 8;58(5):847–855. doi: 10.1016/0092-8674(89)90937-9. [DOI] [PubMed] [Google Scholar]
- Begley G. S., Hansen D. E., Jacobson G. R., Knowles J. R. Stereochemical course of the reactions catalyzed by the bacterial phosphoenolpyruvate:glucose phosphotransferase system. Biochemistry. 1982 Oct 26;21(22):5552–5556. doi: 10.1021/bi00265a026. [DOI] [PubMed] [Google Scholar]
- Bouvet O. M., Grimont P. A. Diversity of the phosphoenolpyruvate/glucose phosphotransferase system in the Enterobacteriaceae. Ann Inst Pasteur Microbiol. 1987 Jan-Feb;138(1):3–13. doi: 10.1016/0769-2609(87)90048-2. [DOI] [PubMed] [Google Scholar]
- Byrne C. R., Monroe R. S., Ward K. A., Kredich N. M. DNA sequences of the cysK regions of Salmonella typhimurium and Escherichia coli and linkage of the cysK regions to ptsH. J Bacteriol. 1988 Jul;170(7):3150–3157. doi: 10.1128/jb.170.7.3150-3157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Reuse H., Danchin A. The ptsH, ptsI, and crr genes of the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system: a complex operon with several modes of transcription. J Bacteriol. 1988 Sep;170(9):3827–3837. doi: 10.1128/jb.170.9.3827-3837.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Reuse H., Lévy S., Zeng G., Danchin A. Genetics of the PTS components in Escherichia coli K-12. FEMS Microbiol Rev. 1989 Jun;5(1-2):61–67. doi: 10.1016/0168-6445(89)90009-0. [DOI] [PubMed] [Google Scholar]
- Erni B. Glucose transport in Escherichia coli. FEMS Microbiol Rev. 1989 Jun;5(1-2):13–23. doi: 10.1016/0168-6445(89)90004-1. [DOI] [PubMed] [Google Scholar]
- Erni B., Zanolari B. Glucose-permease of the bacterial phosphotransferase system. Gene cloning, overproduction, and amino acid sequence of enzyme IIGlc. J Biol Chem. 1986 Dec 15;261(35):16398–16403. [PubMed] [Google Scholar]
- Erni B., Zanolari B., Kocher H. P. The mannose permease of Escherichia coli consists of three different proteins. Amino acid sequence and function in sugar transport, sugar phosphorylation, and penetration of phage lambda DNA. J Biol Chem. 1987 Apr 15;262(11):5238–5247. [PubMed] [Google Scholar]
- Kofoid E. C., Parkinson J. S. Transmitter and receiver modules in bacterial signaling proteins. Proc Natl Acad Sci U S A. 1988 Jul;85(14):4981–4985. doi: 10.1073/pnas.85.14.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J., Auburger A. M., Mayer R., Pecher A. The phosphoenolpyruvate-dependent carbohydrate: phosphotransferase system enzymes II as chemoreceptors in chemotaxis of Escherichia coli K 12. Mol Gen Genet. 1981;183(1):163–170. doi: 10.1007/BF00270156. [DOI] [PubMed] [Google Scholar]
- Levy S., Danchin A. Phylogeny of metabolic pathways: O-acetylserine sulphydrylase A is homologous to the tryptophan synthase beta subunit. Mol Microbiol. 1988 Nov;2(6):777–783. doi: 10.1111/j.1365-2958.1988.tb00089.x. [DOI] [PubMed] [Google Scholar]
- Lévy S., Zeng G. Q., Danchin A. Cyclic AMP synthesis in Escherichia coli strains bearing known deletions in the pts phosphotransferase operon. Gene. 1990 Jan 31;86(1):27–33. doi: 10.1016/0378-1119(90)90110-d. [DOI] [PubMed] [Google Scholar]
- Mattoo R. L., Waygood E. B. Determination of the levels of HPr and enzyme I of the phosphoenolpyruvate-sugar phosphotransferase system in Escherichia coli and Salmonella typhimurium. Can J Biochem Cell Biol. 1983 Jan;61(1):29–37. doi: 10.1139/o83-005. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rephaeli A. W., Saier M. H., Jr Regulation of genes coding for enzyme constituents of the bacterial phosphotransferase system. J Bacteriol. 1980 Feb;141(2):658–663. doi: 10.1128/jb.141.2.658-663.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman S., Meadow N. D. Signal transduction by the bacterial phosphotransferase system. Diauxie and the crr gene (J. Monod revisited). J Biol Chem. 1990 Feb 25;265(6):2993–2996. [PubMed] [Google Scholar]
- Roy A., Danchin A. The cya locus of Escherichia coli K12: organization and gene products. Mol Gen Genet. 1982;188(3):465–471. doi: 10.1007/BF00330050. [DOI] [PubMed] [Google Scholar]
- Roy A., Haziza C., Danchin A. Regulation of adenylate cyclase synthesis in Escherichia coli: nucleotide sequence of the control region. EMBO J. 1983;2(5):791–797. doi: 10.1002/j.1460-2075.1983.tb01502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffen D. W., Presper K. A., Doering T. L., Roseman S. Sugar transport by the bacterial phosphotransferase system. Molecular cloning and structural analysis of the Escherichia coli ptsH, ptsI, and crr genes. J Biol Chem. 1987 Nov 25;262(33):16241–16253. [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U. Coordinate regulation of adenylate cyclase and carbohydrate permeases by the phosphoenolpyruvate:sugar phosphotransferase system in Salmonella typhimurium. J Biol Chem. 1975 Sep 10;250(17):7078–7080. [PubMed] [Google Scholar]
- Saier M. H., Jr Protein phosphorylation and allosteric control of inducer exclusion and catabolite repression by the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Microbiol Rev. 1989 Mar;53(1):109–120. doi: 10.1128/mr.53.1.109-120.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Roseman S. Sugar transport. 2nducer exclusion and regulation of the melibiose, maltose, glycerol, and lactose transport systems by the phosphoenolpyruvate:sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6606–6615. [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Waygood E. B., Meadow N. D., Postma P. W., Roseman S. Sugar transport by the bacterial phosphotransferase system. The glucose receptors of the Salmonella typhimurium phosphotransferase system. J Biol Chem. 1982 Dec 10;257(23):14543–14552. [PubMed] [Google Scholar]
- Vogler A. P., Broekhuizen C. P., Schuitema A., Lengeler J. W., Postma P. W. Suppression of IIIGlc-defects by enzymes IINag and IIBgl of the PEP:carbohydrate phosphotransferase system. Mol Microbiol. 1988 Nov;2(6):719–726. doi: 10.1111/j.1365-2958.1988.tb00082.x. [DOI] [PubMed] [Google Scholar]
- Weston L. A., Kadner R. J. Role of uhp genes in expression of the Escherichia coli sugar-phosphate transport system. J Bacteriol. 1988 Aug;170(8):3375–3383. doi: 10.1128/jb.170.8.3375-3383.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. L., Goldrick D., Hong J. S. Identification of the products and nucleotide sequences of two regulatory genes involved in the exogenous induction of phosphoglycerate transport in Salmonella typhimurium. J Bacteriol. 1988 Sep;170(9):4299–4303. doi: 10.1128/jb.170.9.4299-4303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


