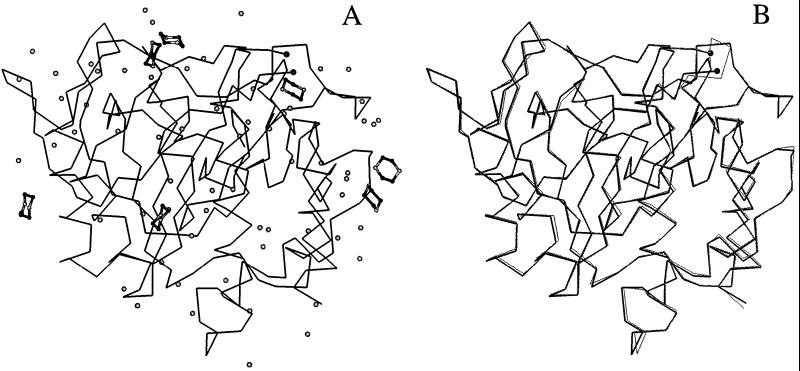
Figure 2.
(A) Cα trace of the protein structure of subtilisin Carlsberg in anhydrous dioxane (black lines) with bound water (gray balls) and dioxane (balls-and-sticks: C atoms in black and O atoms in gray) molecules. (B) Superimposition of the Cα traces of subtilisin in water (thin gray lines) and in anhydrous dioxane (thicker black lines). The rms shifts of the Cα atoms and of the main-chain atoms are 0.31 Å and 0.33 Å, respectively. Ser-159 and Gly-160 are not included in the model of subtilisin in dioxane because of uncertainties in their location; the Cα atoms of Asn-158 and Ser-161 are shown as balls (upper right).