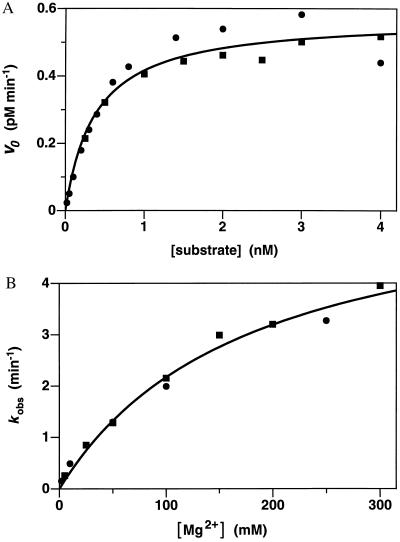
Figure 3.
Catalytic activity of the 10-23 DNA enzyme under multiple turnover conditions. (A) Initial velocities were measured over the first 10% of the reaction, using a fixed concentration of enzyme (0.004 nM) and varying concentrations of substrate (0.02–4 nM). The 17-mer RNA substrate, corresponding to the start codon region of HIV-1 gag/pol mRNA, was prepared by in vitro transcription. Reaction conditions: 2 mM MgCl2 and 150 mM NaCl (pH 7.5) at 37°C. Data from two independent experiments are shown and were fit to the Michaelis–Menten equation: v = kcat [E]/(Km + [S]). (B) Catalytic rates were determined in the presence of saturating substrate (100 nM) and varying concentrations of MgCl2 (2–300 mM). Reaction conditions were otherwise as above. Data from two independent experiments are shown and were fit to the Michaelis–Menten equation to obtain the apparent Km for Mg2+.