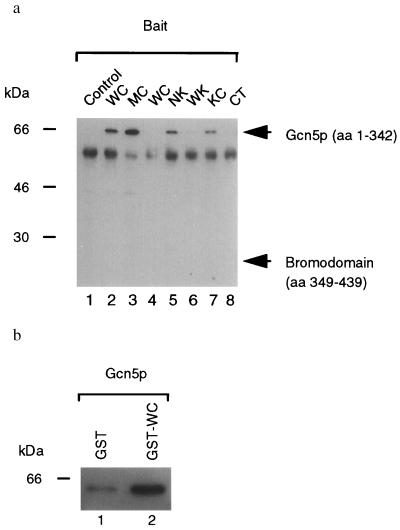
Figure 2.
Physical association of Ire1p and Gcn5p in vivo and in vitro. (a) Coimmunoprecipitations of Gcn5p from yeast cell lysates. Lysates from cells coexpressing either B42–HA–Gcn5 (amino acids 1–342; lanes 1–3 and 5–8) or B42-HA-Gcn5 BD (amino acids 349–439; lane 4) as prey and LexADB (lane 1), LexADB–WC (lanes 2 and 4), LexADB–MC (lane 3), LexADB–NK (lane 5), LexADB–WK (lane 6), LexADB–KC (lane 7), and LexADB–CT (lane 8) as bait were immunoprecipitated with anti-LexA antibodies (generously provided by Erica Golemis, Fox Chase Cancer Center, Philadelphia), and immunoprecipitated proteins were analyzed by Western blotting with anti-HA antibody (Boehringer Mannheim). Expected migration of the prey proteins are indicated. The 65-kDa species is the size expected for the B42 transcriptional activator–HA–Gcn5p (residues 1–342) fusion protein. The 55-kDa background band is rabbit IgG heavy chain. WC, wild-type cytoplasmic/nucleoplasmic domain; MC, K702A mutant cytoplasmic/nucleoplasmic domain; NK, N-linker plus kinase domain; WK, wild-type kinase domain; KC, kinase plus C-terminal tail; CT, C-terminal tail. (b) In vitro binding assay. Either GST (lane 1) or GST–WC (lane 2) proteins were used to immunoabsorb B42–HA–Gcn5p and absorbed proteins analyzed by Western blotting with anti-HA antibody (Boehringer Mannheim). WC, wild-type cytoplasmic/nucleoplasmic domain.