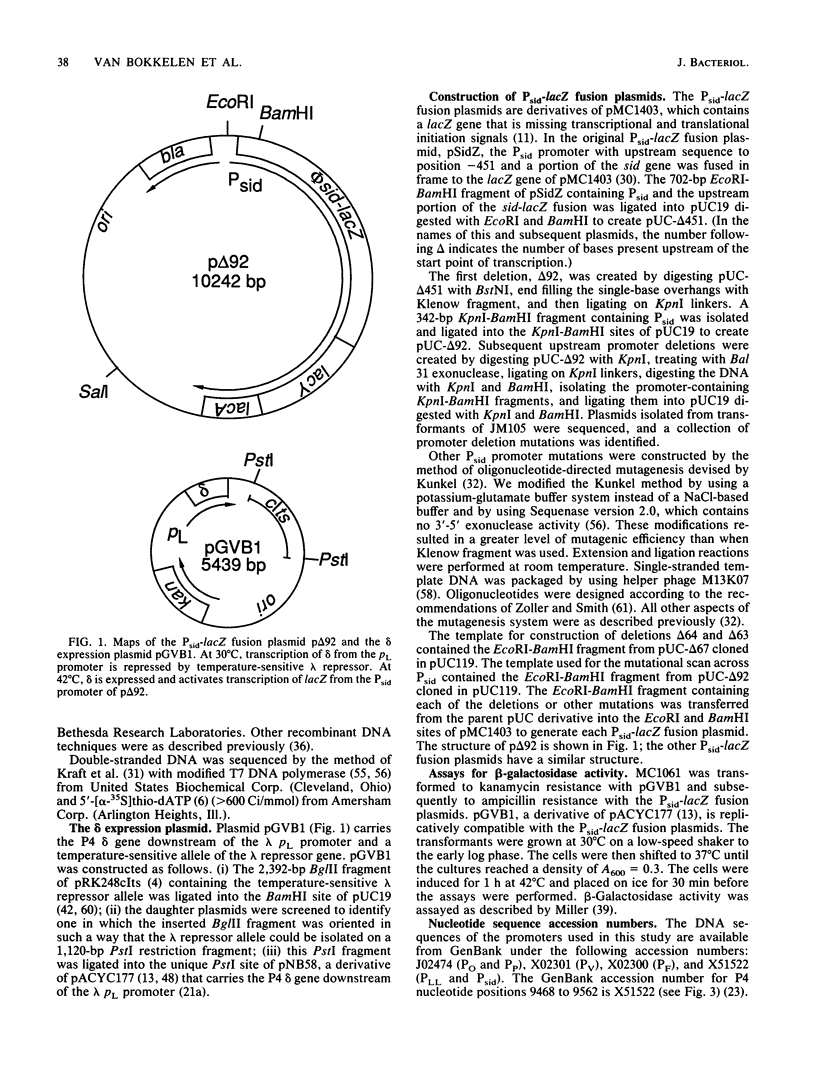
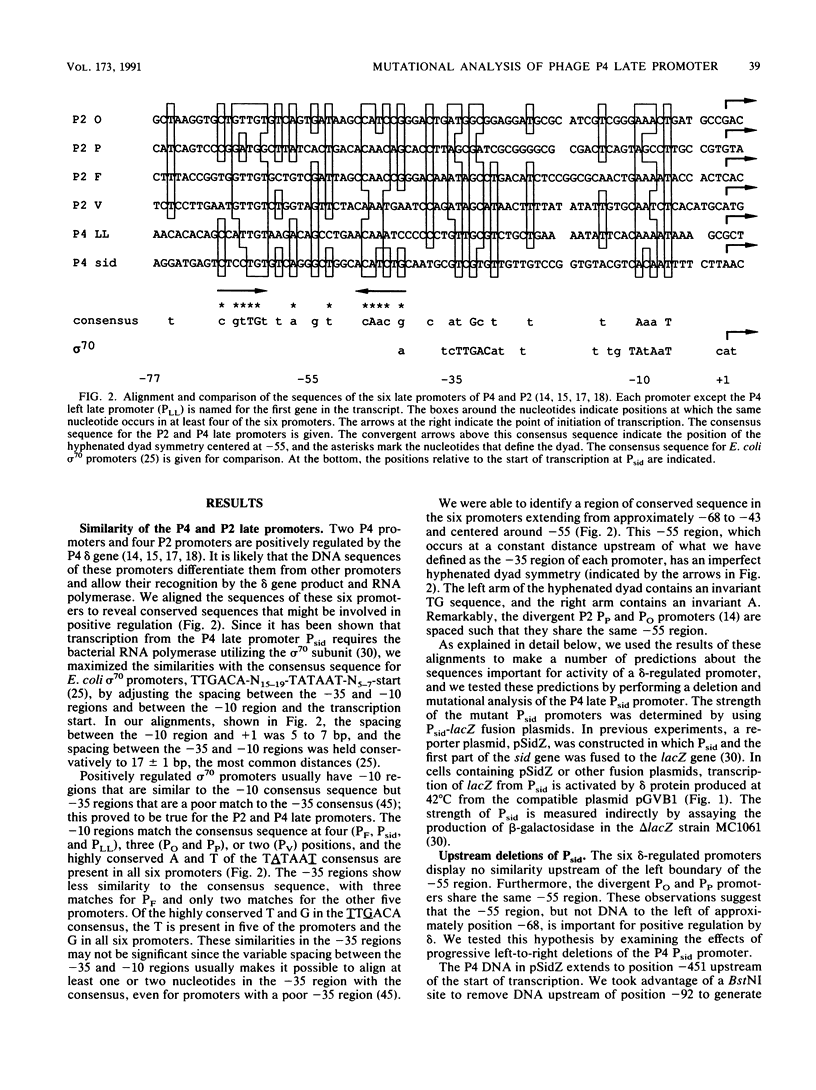
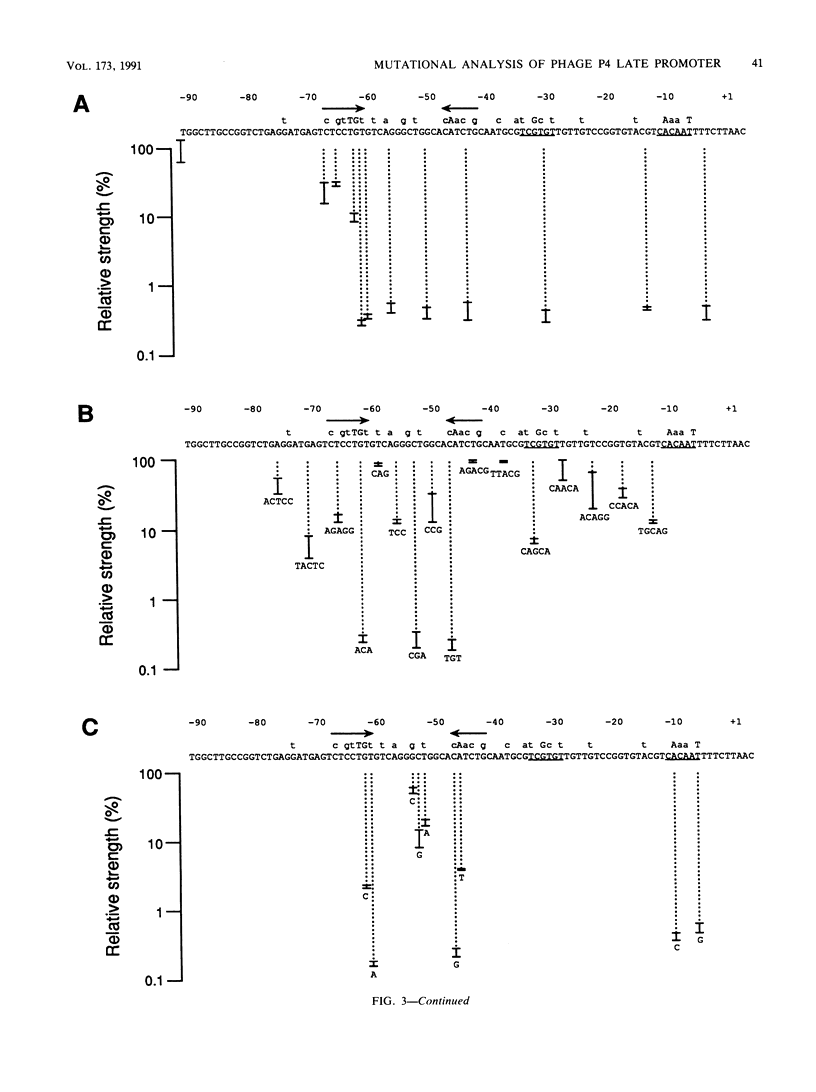
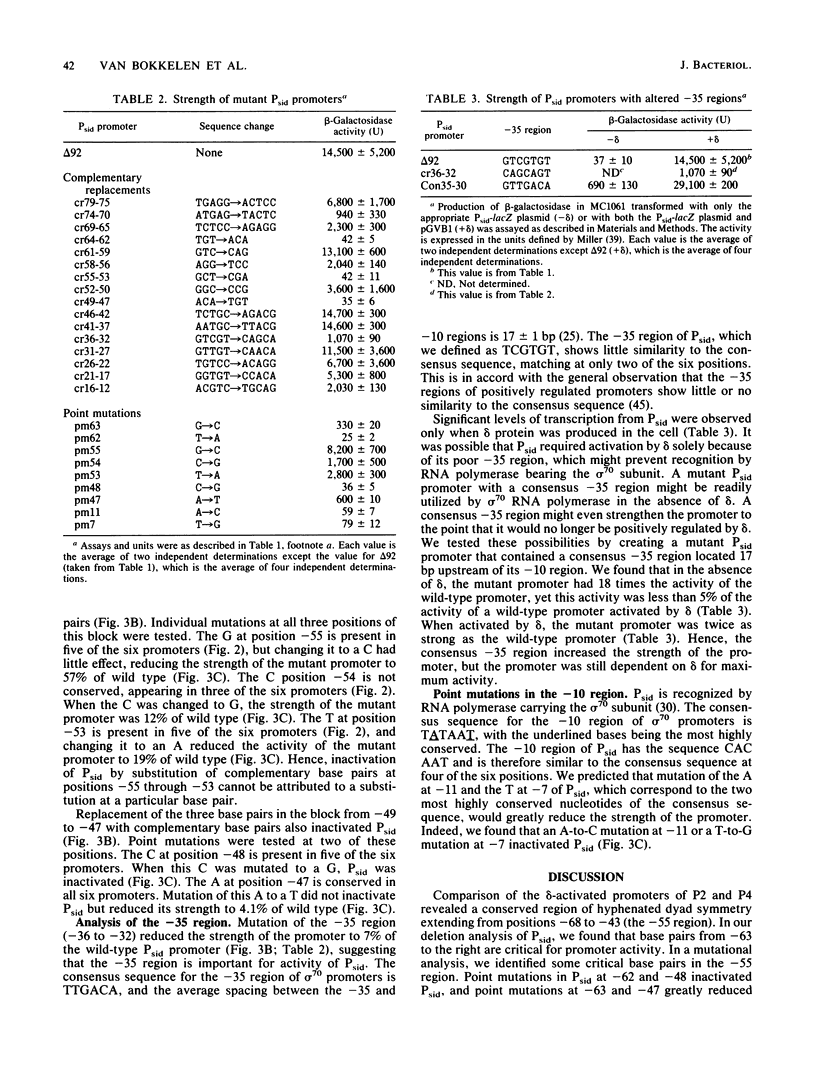
Abstract
Transcription from the late Psid promoter of satellite bacteriophage P4 is dependent on the bacterial RNA polymerase carrying the sigma 70 subunit and is positively regulated by the product of the P4 delta gene or the ogr gene of helper bacteriophage P2. Through deletion and mutational analyses of the Psid promoter, we identified mutations in the -10 region and in a region of hyphenated dyad symmetry centered around position -55 that inactivate Psid. Most of these mutations alter base pairs that are highly conserved in the five other delta-activated P4 and P2 late promoters. We propose that the P4 delta and P2 ogr gene products bind the -55 region of the P4 and P2 late promoters.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal A. K., Rodgers D. W., Drottar M., Ptashne M., Harrison S. C. Recognition of a DNA operator by the repressor of phage 434: a view at high resolution. Science. 1988 Nov 11;242(4880):899–907. doi: 10.1126/science.3187531. [DOI] [PubMed] [Google Scholar]
- Austin S., Henderson N., Dixon R. Requirements for transcriptional activation in vitro of the nitrogen-regulated glnA and nifLA promoters from Klebsiella pneumoniae: dependence on activator concentration. Mol Microbiol. 1987 Jul;1(1):92–100. doi: 10.1111/j.1365-2958.1987.tb00532.x. [DOI] [PubMed] [Google Scholar]
- Barthelemy I., Salas M. Characterization of a new prokaryotic transcriptional activator and its DNA recognition site. J Mol Biol. 1989 Jul 20;208(2):225–232. doi: 10.1016/0022-2836(89)90384-7. [DOI] [PubMed] [Google Scholar]
- Bernard H. U., Remaut E., Hershfield M. V., Das H. K., Helinski D. R., Yanofsky C., Franklin N. Construction of plasmid cloning vehicles that promote gene expression from the bacteriophage lambda pL promoter. Gene. 1979 Jan;5(1):59–76. doi: 10.1016/0378-1119(79)90092-1. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkeland N. K., Christie G. E., Lindqvist B. H. Directed mutagenesis of the bacteriophage P2 ogr gene defines an essential function. Gene. 1988 Dec 20;73(2):327–335. doi: 10.1016/0378-1119(88)90497-0. [DOI] [PubMed] [Google Scholar]
- Birkeland N. K., Lindquist B. H. Coliphage P2 late control gene ogr. DNA sequence and product identification. J Mol Biol. 1986 Apr 5;188(3):487–490. doi: 10.1016/0022-2836(86)90170-1. [DOI] [PubMed] [Google Scholar]
- Bölker M., Wulczyn F. G., Kahmann R. Role of bacteriophage Mu C protein in activation of the mom gene promoter. J Bacteriol. 1989 Apr;171(4):2019–2027. doi: 10.1128/jb.171.4.2019-2027.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie G. E., Calendar R. Bacteriophage P2 late promoters. II. Comparison of the four late promoter sequences. J Mol Biol. 1985 Feb 5;181(3):373–382. doi: 10.1016/0022-2836(85)90226-8. [DOI] [PubMed] [Google Scholar]
- Christie G. E., Calendar R. Bacteriophage P2 late promoters. Transcription initiation sites for two late mRNAs. J Mol Biol. 1983 Jul 15;167(4):773–790. doi: 10.1016/s0022-2836(83)80110-7. [DOI] [PubMed] [Google Scholar]
- Christie G. E., Calendar R. Interactions between satellite bacteriophage P4 and its helpers. Annu Rev Genet. 1990;24:465–490. doi: 10.1146/annurev.ge.24.120190.002341. [DOI] [PubMed] [Google Scholar]
- Christie G. E., Haggård-Ljungquist E., Feiwell R., Calendar R. Regulation of bacteriophage P2 late-gene expression: the ogr gene. Proc Natl Acad Sci U S A. 1986 May;83(10):3238–3242. doi: 10.1073/pnas.83.10.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale E. C., Christie G. E., Calendar R. Organization and expression of the satellite bacteriophage P4 late gene cluster. J Mol Biol. 1986 Dec 20;192(4):793–803. doi: 10.1016/0022-2836(86)90029-x. [DOI] [PubMed] [Google Scholar]
- Dehó G., Zangrossi S., Ghisotti D., Sironi G. Alternative promoters in the development of bacteriophage plasmid P4. J Virol. 1988 May;62(5):1697–1704. doi: 10.1128/jvi.62.5.1697-1704.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Fujiki H., Palm P., Zillig W., Calendar R., Sunshine M. Identification of a mutation within the structural gene for the a subunit of DNA-dependent RNA polymerase of E. coli. Mol Gen Genet. 1976 Apr 23;145(1):19–22. doi: 10.1007/BF00331552. [DOI] [PubMed] [Google Scholar]
- Gartenberg M. R., Crothers D. M. DNA sequence determinants of CAP-induced bending and protein binding affinity. Nature. 1988 Jun 30;333(6176):824–829. doi: 10.1038/333824a0. [DOI] [PubMed] [Google Scholar]
- Grambow N. J., Birkeland N. K., Anders D. L., Christie G. E. Deletion analysis of a bacteriophage P2 late promoter. Gene. 1990 Oct 30;95(1):9–15. doi: 10.1016/0378-1119(90)90407-i. [DOI] [PubMed] [Google Scholar]
- Halling C., Calendar R. Bacteriophage P2 ogr and P4 delta genes act independently and are essential for P4 multiplication. J Bacteriol. 1990 Jul;172(7):3549–3558. doi: 10.1128/jb.172.7.3549-3558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling C., Calendar R., Christie G. E., Dale E. C., Dehò G., Finkel S., Flensburg J., Ghisotti D., Kahn M. L., Lane K. B. DNA sequence of satellite bacteriophage P4. Nucleic Acids Res. 1990 Mar 25;18(6):1649–1649. doi: 10.1093/nar/18.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling C., Sunshine M. G., Lane K. B., Six E. W., Calendar R. A mutation of the transactivation gene of satellite bacteriophage P4 that suppresses the rpoA109 mutation of Escherichia coli. J Bacteriol. 1990 Jul;172(7):3541–3548. doi: 10.1128/jb.172.7.3541-3548.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. S., Wulff D. L., Rosenberg M. Bacteriophage lambda protein cII binds promoters on the opposite face of the DNA helix from RNA polymerase. Nature. 1983 Aug 25;304(5928):703–708. doi: 10.1038/304703a0. [DOI] [PubMed] [Google Scholar]
- Hochschild A., Irwin N., Ptashne M. Repressor structure and the mechanism of positive control. Cell. 1983 Feb;32(2):319–325. doi: 10.1016/0092-8674(83)90451-8. [DOI] [PubMed] [Google Scholar]
- Jordan S. R., Pabo C. O. Structure of the lambda complex at 2.5 A resolution: details of the repressor-operator interactions. Science. 1988 Nov 11;242(4880):893–899. doi: 10.1126/science.3187530. [DOI] [PubMed] [Google Scholar]
- Kalionis B., Pritchard M., Egan J. B. Control of gene expression in the P2-related temperate coliphages. IV. Concerning the late control gene and control of its transcription. J Mol Biol. 1986 Sep 20;191(2):211–220. doi: 10.1016/0022-2836(86)90258-5. [DOI] [PubMed] [Google Scholar]
- Keener J., Dale E. C., Kustu S., Calendar R. In vitro transcription from the late promoter of bacteriophage P4. J Bacteriol. 1988 Aug;170(8):3543–3546. doi: 10.1128/jb.170.8.3543-3546.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft R., Tardiff J., Krauter K. S., Leinwand L. A. Using mini-prep plasmid DNA for sequencing double stranded templates with Sequenase. Biotechniques. 1988 Jun;6(6):544-6, 549. [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Francklyn C., Hamilton E. P. Arabinose-induced binding of AraC protein to araI2 activates the araBAD operon promoter. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8814–8818. doi: 10.1073/pnas.84.24.8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. C., Christie G. E. Purification and properties of the bacteriophage P2 ogr gene product. A prokaryotic zinc-binding transcriptional activator. J Biol Chem. 1990 May 5;265(13):7472–7477. [PubMed] [Google Scholar]
- Maeda S., Ozawa Y., Mizuno T., Mizushima S. Stereospecific positioning of the cis-acting sequence with respect to the canonical promoter is required for activation of the ompC gene by a positive regulator, OmpR, in Escherichia coli. J Mol Biol. 1988 Aug 5;202(3):433–441. doi: 10.1016/0022-2836(88)90276-8. [DOI] [PubMed] [Google Scholar]
- Margolin W., Rao G., Howe M. M. Bacteriophage Mu late promoters: four late transcripts initiate near a conserved sequence. J Bacteriol. 1989 Apr;171(4):2003–2018. doi: 10.1128/jb.171.4.2003-2018.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M., Hanish J., Nelson M., Patel Y. KGB: a single buffer for all restriction endonucleases. Nucleic Acids Res. 1988 Jan 11;16(1):364–364. doi: 10.1093/nar/16.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Taylor R. K., Mekalanos J. J. Cholera toxin transcriptional activator toxR is a transmembrane DNA binding protein. Cell. 1987 Jan 30;48(2):271–279. doi: 10.1016/0092-8674(87)90430-2. [DOI] [PubMed] [Google Scholar]
- Ninfa A. J., Reitzer L. J., Magasanik B. Initiation of transcription at the bacterial glnAp2 promoter by purified E. coli components is facilitated by enhancers. Cell. 1987 Sep 25;50(7):1039–1046. doi: 10.1016/0092-8674(87)90170-x. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- O'Halloran T. V., Frantz B., Shin M. K., Ralston D. M., Wright J. G. The MerR heavy metal receptor mediates positive activation in a topologically novel transcription complex. Cell. 1989 Jan 13;56(1):119–129. doi: 10.1016/0092-8674(89)90990-2. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z., Schevitz R. W., Zhang R. G., Lawson C. L., Joachimiak A., Marmorstein R. Q., Luisi B. F., Sigler P. B. Crystal structure of trp repressor/operator complex at atomic resolution. Nature. 1988 Sep 22;335(6188):321–329. doi: 10.1038/335321a0. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Vidal-Ingigliardi D., Richet E. A complex nucleoprotein structure involved in activation of transcription of two divergent Escherichia coli promoters. J Mol Biol. 1989 Feb 5;205(3):471–485. doi: 10.1016/0022-2836(89)90218-0. [DOI] [PubMed] [Google Scholar]
- Ren Y. L., Garges S., Adhya S., Krakow J. S. Cooperative DNA binding of heterologous proteins: evidence for contact between the cyclic AMP receptor protein and RNA polymerase. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4138–4142. doi: 10.1073/pnas.85.12.4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose R. E. The nucleotide sequence of pACYC177. Nucleic Acids Res. 1988 Jan 11;16(1):356–356. doi: 10.1093/nar/16.1.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Six E. W., Klug C. A. Bacteriophage P4: a satellite virus depending on a helper such as prophage P2. Virology. 1973 Feb;51(2):327–344. doi: 10.1016/0042-6822(73)90432-7. [DOI] [PubMed] [Google Scholar]
- Six E. W. The helper dependence of satellite bacteriophage P4: which gene functions of bacteriophage P2 are needed by P4? Virology. 1975 Sep;67(1):249–263. doi: 10.1016/0042-6822(75)90422-5. [DOI] [PubMed] [Google Scholar]
- Souza L., Calendar R., Six E. W., Lindqvist B. H. A transactivation mutant of satellite phage P4. Virology. 1977 Aug;81(1):81–90. doi: 10.1016/0042-6822(77)90060-5. [DOI] [PubMed] [Google Scholar]
- Storz G., Tartaglia L. A., Ames B. N. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990 Apr 13;248(4952):189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- Straney D. C., Straney S. B., Crothers D. M. Synergy between Escherichia coli CAP protein and RNA polymerase in the lac promoter open complex. J Mol Biol. 1989 Mar 5;206(1):41–57. doi: 10.1016/0022-2836(89)90522-6. [DOI] [PubMed] [Google Scholar]
- Sunshine M. G., Sauer B. A bacterial mutation blocking P2 phage late gene expression. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2770–2774. doi: 10.1073/pnas.72.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. Selective inactivation of the exonuclease activity of bacteriophage T7 DNA polymerase by in vitro mutagenesis. J Biol Chem. 1989 Apr 15;264(11):6447–6458. [PubMed] [Google Scholar]
- Tobin J. F., Schleif R. F. Purification and properties of RhaR, the positive regulator of the L-rhamnose operons of Escherichia coli. J Mol Biol. 1990 Jan 5;211(1):75–89. doi: 10.1016/0022-2836(90)90012-B. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Wolberger C., Dong Y. C., Ptashne M., Harrison S. C. Structure of a phage 434 Cro/DNA complex. Nature. 1988 Oct 27;335(6193):789–795. doi: 10.1038/335789a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]


