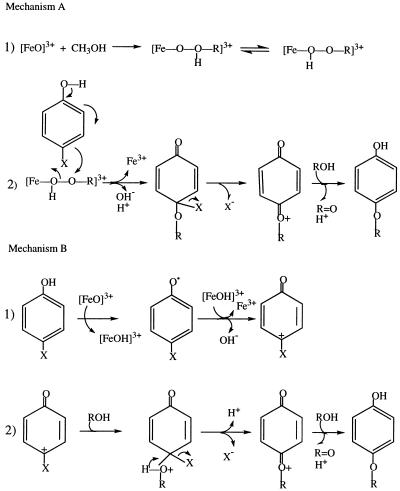
Figure 3.
Proposed mechanisms for the formation of the alkoxyphenols from the MP8-mediated dehalogenation of halophenols in alcoholic solvents. (A) Step 1: The formation of a MP8-alkylperoxide intermediate resulting from the reaction of compound I type with the alcohol. Step 2: The reaction of this alkylperoxide intermediate with the electron-rich centers of a halophenol which upon electronic rearrangement leads to the elimination of the halogen as an anion and the formation of an intermediate. This intermediate is further reduced by a solvent molecule resulting in the formation of the alkoxy product and an aldehyde. (B) An alternative mechanism for the product formation. Step 1: The formation of carbocation intermediates through the two consecutive one-electron oxidations of a halophenol by compounds I and II of the heme catalyst. Step 2: The nucleophilic attack of solvent molecules on these carbocation derivatives, followed by the release of the halogen as an anion and the formation of the same intermediate as in A, step 2, whose subsequent reduction by a solvent molecule leads to the final product formation.