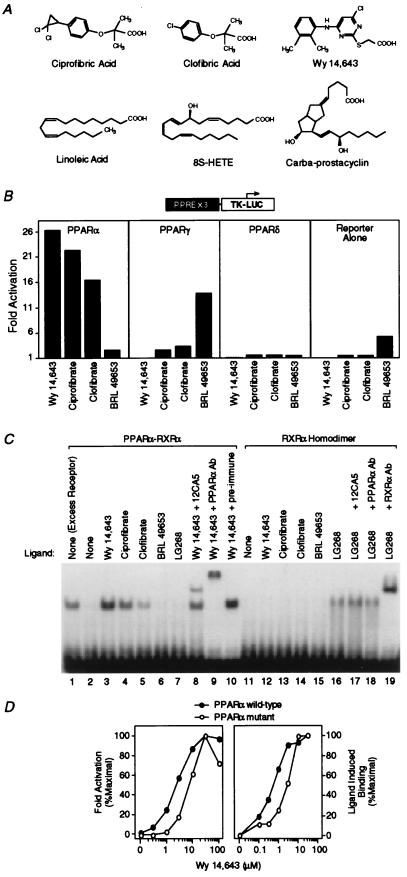
Figure 1.
Hypolipidemic fibrates are ligands for PPARα. (A) Chemical structures of some compounds that we demonstrate to be ligands for PPARα or -δ. (B) Fibrates selectively activate PPARα in a cell-based transient transfection assay. Cells were treated with the following concentrations of each compound: 5 μM Wy 14,643, 300 μM ciprofibrate, 300 μM clofibrate, and 1 μM BRL 49653. (C) Fibrates selectively promote binding of PPARα–RXRα heterodimers to labeled DNA in an electrophoretic mobility shift assay. Compounds were added at the following concentrations: 5 μM Wy 14,643, 100 μM ciprofibrate, 1,000 μM clofibrate, 1 μM BRL 49653, and 1 μM LG268. In lane 1 (excess receptor), the amounts of PPARα and RXRα were increased to 0.6 μl and 0.5 μl, respectively. Where indicated, 1 μl of antibody was added to the reaction. (D) Comparison of the dose response profile of wild-type PPARα with PPARα-G (Glu-282 → Gly) (12) in the transient transfection assay (Left) and the LIC assay (Right). The ligand-induced complex was quantified by phosphorimaging analysis. Ligand-induced binding represents the amount of complex produced at any concentration of ligand minus that produced in the absence of ligand. Maximal induced binding was defined to be 100%; binding observed at other concentrations was normalized to this value.