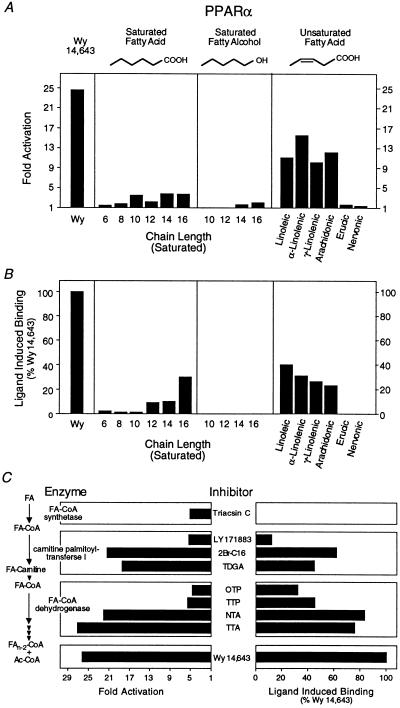
Figure 2.
Long-chain FAs and β-oxidation inhibitors are PPARα ligands. (A) Activation of PPARα by FAs and fatty alcohols. All compounds were added to a final concentration of 30 μM except for Wy 14,643, which was used at 5 μM. (B) Enhancement of PPARα–RXRα heterodimer formation by FAs and fatty alcohols. All compounds were added to a final concentration of 30 μM except for Wy 14,643, which was added to a final concentration of 5 μM. Saturated FAs and fatty alcohols are indicated by their chain length. Unsaturated FAs are as follows: linoleic (cis-Δ9,12-C18:2), α-linolenic (cis-Δ9,12,15-C18:3), γ-linolenic (cis-Δ6,9,12-C18:3), arachidonic (cis-Δ5,8,11,14-C20:4), erucic (cis-Δ13-C22:1), and nervonic (cis-Δ15-C24:1) acids. (C) Inhibitors of β-oxidation activation (Left) and bind (Right) to PPARα. Experiments were performed as described in Fig. 1. Triacsin C (10 μM, Left; 30 μM, Right) was used as an inhibitor of fatty LC-FACS. Inhibitors of carnitine palmitoyltransferase I included LY 171883 (30 μM), 2Br-C16 (5 μM), and tetradecylglycidic acid (TDGA, 5 μM). Fatty-acyl-CoA dehydrogenase was inhibited with octylthioproprionic acid (OTP, 30 μM), tetradecylthioproprionic acid (TTP, 30 μM), nonylthioacetic acid (NTA, 30 μM), and tetradecylthioacetic acid (TTA, 30 μM). Wy 14,643 (5 μM) was included as a positive control.