TABLE 3.
Chemical potential and its components for OPLS and KBFF model urea solutions using Eq. 37
| Mole fraction | 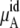 |
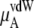 |
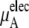 |
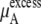 |
μA |
|---|---|---|---|---|---|
| OPLS | |||||
| 0.0007657 | −41.31 | 0.87 | −56.00 | −55.13 | −96.44 |
| 0.03806 | −31.76 | 0.66 | −56.40 | −55.74 | −87.49 |
| 0.08106 | −30.02 | 0.33 | −56.19 | −55.86 | −85.88 |
| 0.1294 | −29.01 | 0.49 | −56.56 | −56.07 | −85.08 |
| 0.1840 | −28.29 | 0.040 | −56.63 | −56.59 | −84.88 |
| 0.2728 | −27.56 | −0.63 | −57.11 | −57.73 | −85.29 |
| 1.00 | −25.78 | −1.29 | −56.43 | −57.72 | −83.50 |
| KBFF | |||||
| 0.0007657 | −41.31 | 2.14 | −46.40 | −44.26 | −85.57 |
| 0.03806 | −31.78 | 1.29 | −45.57 | −44.29 | −76.06 |
| 0.08106 | −30.06 | 0.38 | −44.79 | −44.41 | −74.46 |
| 0.1294 | −29.06 | −0.27 | −44.22 | −44.49 | −73.54 |
| 0.1840 | −28.36 | −1.16 | −43.12 | −44.28 | −72.64 |
| 0.2728 | −27.64 | −2.44 | −41.83 | −44.28 | −71.91 |
| 1.00 | −25.80 | −4.82 | −37.40 | −43.23 | −69.03 |
The quantity 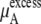 consists of two terms, the vdW part
consists of two terms, the vdW part 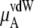 and the electrostatic part
and the electrostatic part 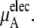
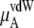 plus
plus 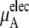 becomes
becomes 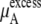 in Eq. 37. The quantity
in Eq. 37. The quantity 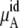 plus
plus 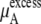 becomes the chemical potential μA. The units of chemical potential are (kJ/mol).
becomes the chemical potential μA. The units of chemical potential are (kJ/mol).
