Abstract
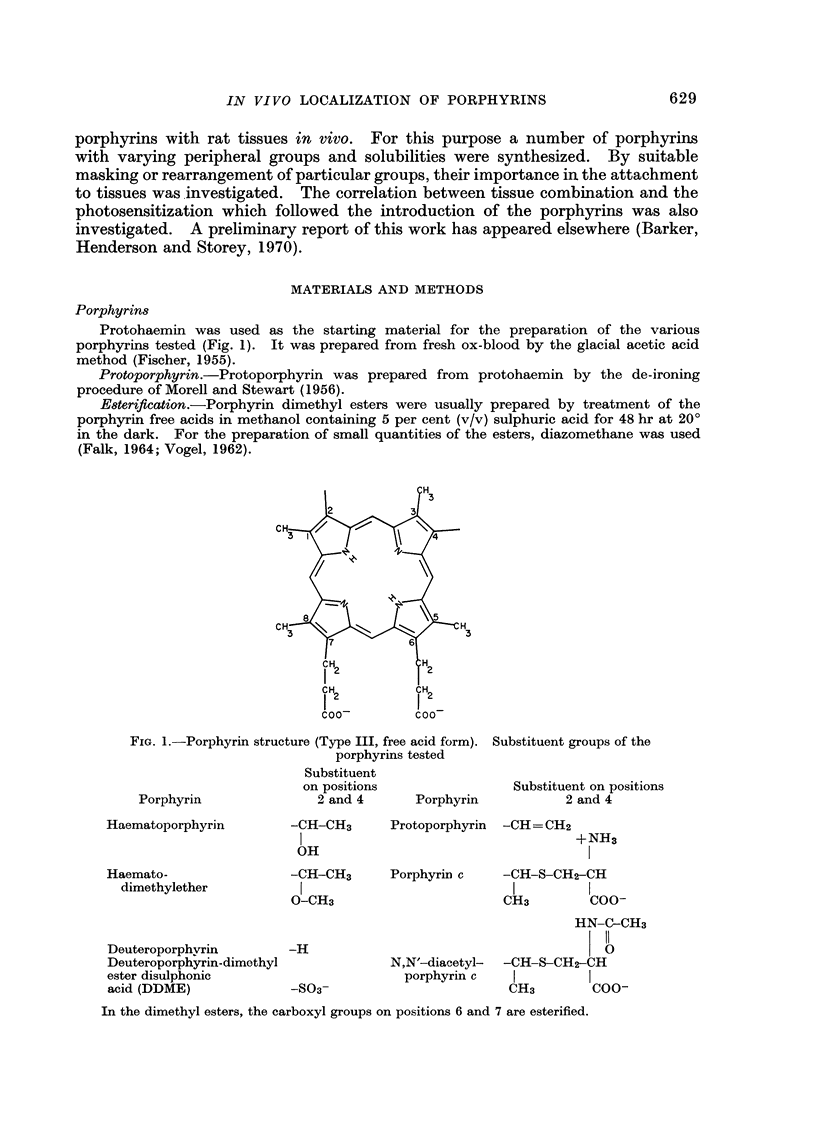
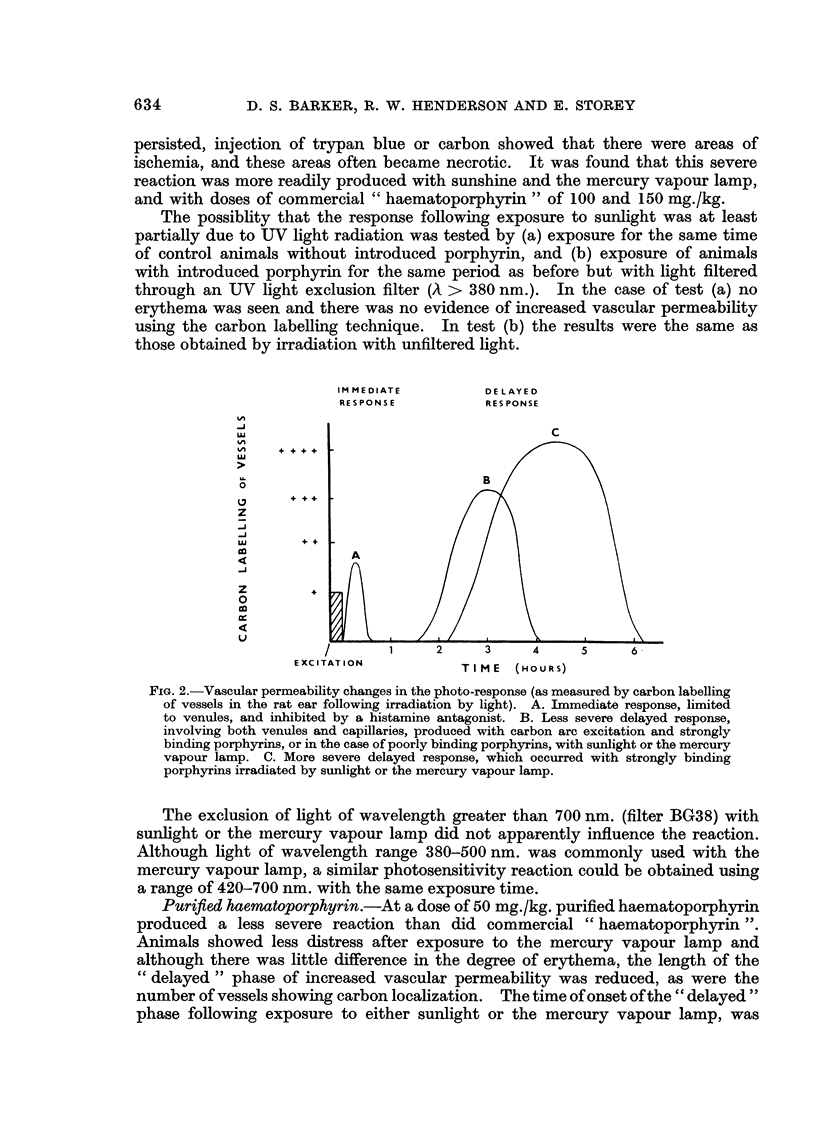
A series of porphyrins with varying side chains was synthesized and tested in the rat for hard and soft tissue binding and photosensitization. It was found that for combination with either type of tissue there was a requirement for groups capable of ionic or hydrogen bonding at more than one edge of the tetrapyrrole nucleus. Thus binding to keratin, collagen and growing bone occurred with purified haematopophyrin, commercial “haematoporphyrin” and haematoporphyrin “derivative”. Little or no binding with these tissues occurred with deuteroporphyrin, deuteroporphyrin dimethyl ester disulphonic acid, haematoporphyrin dimethylether and unexpectedly, porphyrin c and N,N′-diacetyl porphyrin c. Incorporation of iron into the last two named is thought to account for their apparent lack of binding. In the case of growing bone, the same pattern was apparent with the exception that porphyrin c and N,N′-diacetyl porphyrin c were very strong labels. Combination to bone was with the organic rather than with the inorganic component. All soluble porphyrins tested induced a potential photosensitivity on intraperitoneal injection. Using intravenous colloidal carbon as an indicator of an increase in vascular permeability, the photoresponse following excitation with sunlight, mercury vapour lamp or carbon arc was found to be biphasic, with the delayed response altered by dosage and binding ability of porphyrins, and the amount of light absorbed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COTRAN R. S., MAJNO G. THE DELAYED AND PROLONGED VASCULAR LEAKAGE IN INFLAMMATION. I. TOPOGRAPHY OF THE LEAKING VESSELS AFTER THERMAL INJURY. Am J Pathol. 1964 Aug;45:261–281. [PMC free article] [PubMed] [Google Scholar]
- Gregorie H. B., Jr, Horger E. O., Ward J. L., Green J. F., Richards T., Robertson H. C., Jr, Stevenson T. B. Hematoporphyrin-derivative fluorescence in malignant neoplasms. Ann Surg. 1968 Jun;167(6):820–828. doi: 10.1097/00000658-196806000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORELL D. B., STEWART M. The removal of iron from haemins. Aust J Exp Biol Med Sci. 1956 Jun;34(3):211–217. doi: 10.1038/icb.1956.25. [DOI] [PubMed] [Google Scholar]
- Magnus I. A. Photobiological aspects of porphyria. Proc R Soc Med. 1968 Feb;61(2):196–198. [PMC free article] [PubMed] [Google Scholar]
- Neuberger A. Biochemical basis and regulatory mechanisms of porphyrin synthesis. Proc R Soc Med. 1968 Feb;61(2):191–193. [PMC free article] [PubMed] [Google Scholar]
- Prescott G. H., Mitchell D. F., Fahmy H. Procion dyes as matrix markers in growing bone and teeth. Am J Phys Anthropol. 1968 Sep;29(2):219–224. doi: 10.1002/ajpa.1330290215. [DOI] [PubMed] [Google Scholar]
- RUDMAN D., KENDALL F. E. Bile acid content of human serum. II. The binding of cholanic acids by human plasma proteins. J Clin Invest. 1957 Apr;36(4):538–542. doi: 10.1172/JCI103451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUNGE W., WATSON C. J. Experimental production of skin lesions in human cutaneous porphyria. Proc Soc Exp Biol Med. 1962 Apr;109:809–811. doi: 10.3181/00379727-109-27342. [DOI] [PubMed] [Google Scholar]
- Steele R. H., Wilhelm D. L. The inflammatory reaction in chemical injury. I. Increased vascular permeability and erythema induced by various chemicals. Br J Exp Pathol. 1966 Dec;47(6):612–623. [PMC free article] [PubMed] [Google Scholar]
- WALTER R. I. Potentiometric studies of a sulfonated iron porphyrin. J Biol Chem. 1952 May;196(1):151–174. [PubMed] [Google Scholar]


