Abstract
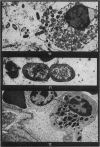
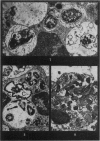
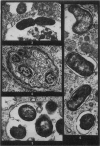
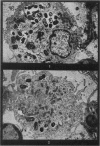
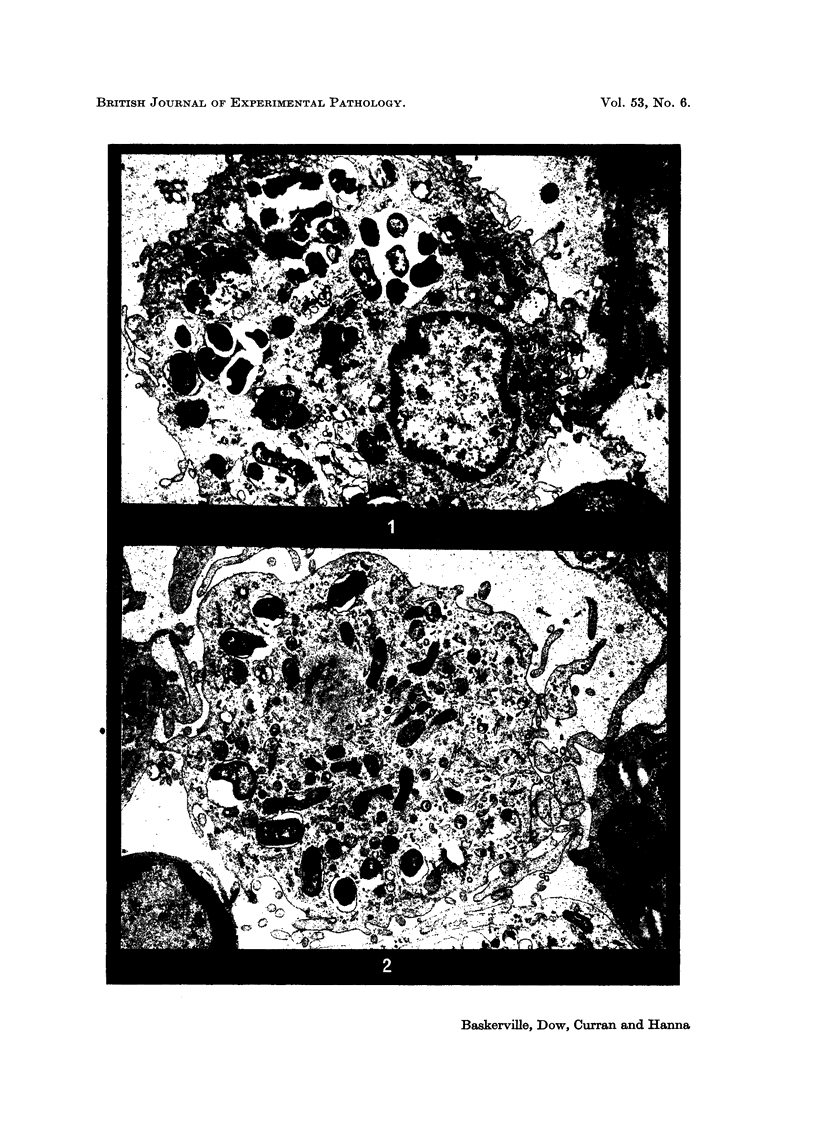
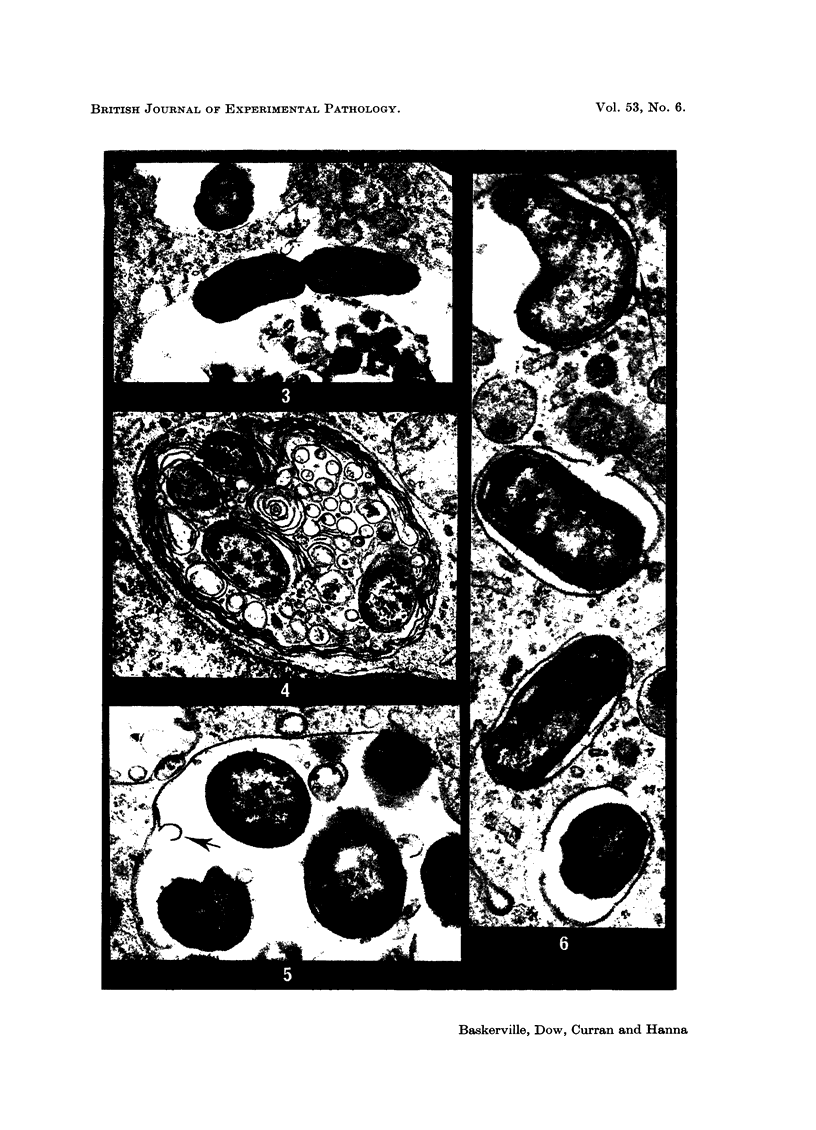
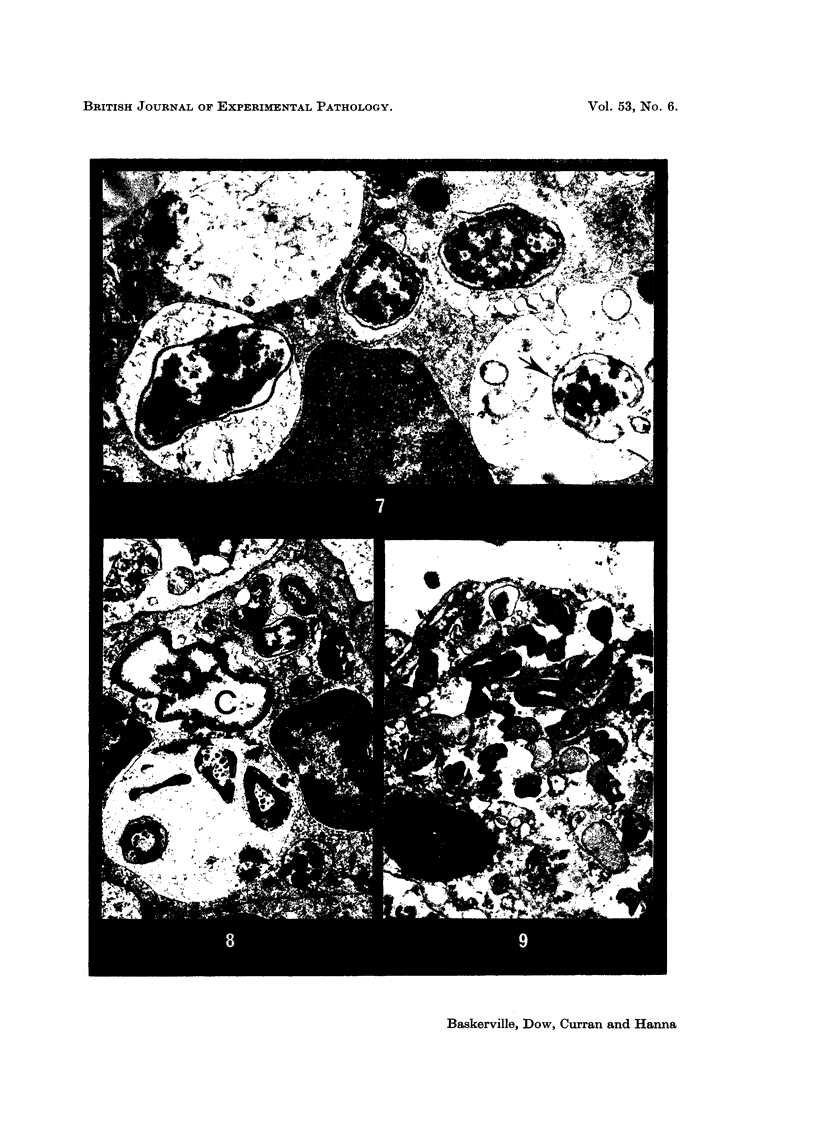
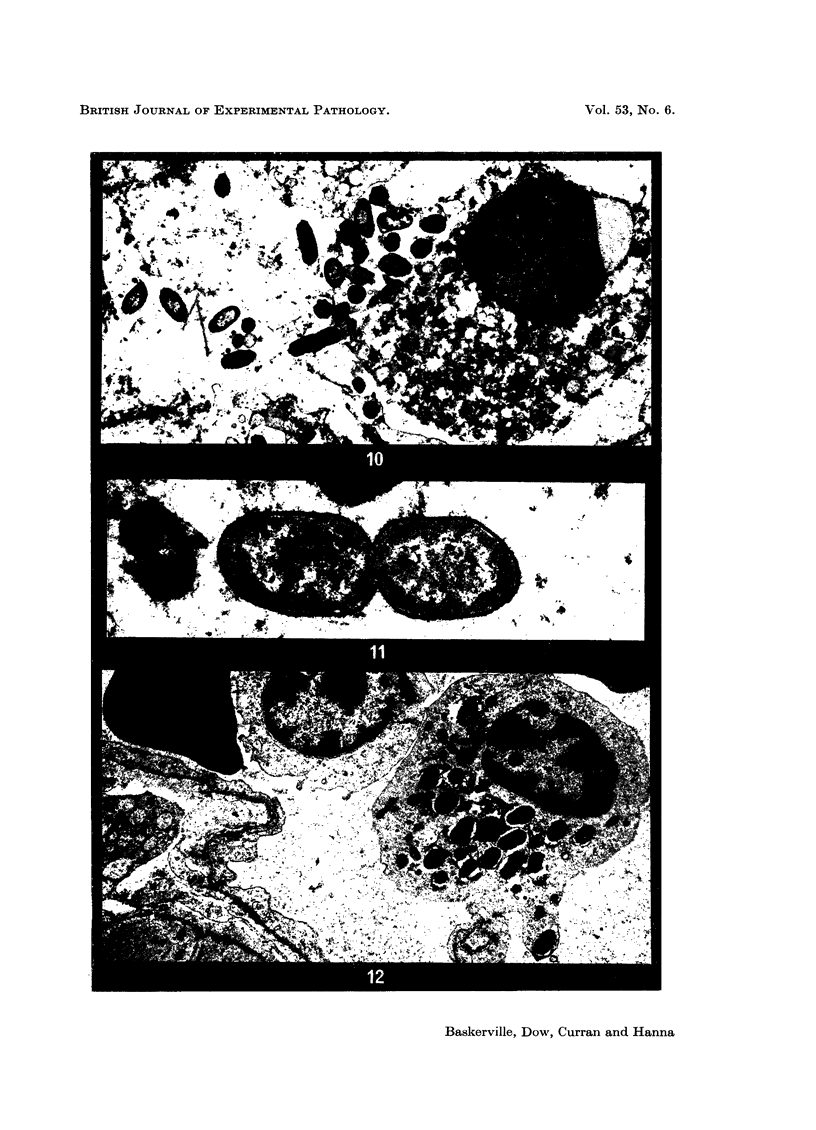
Phagocytosis of virulent Salmonella cholerae-suis in the lungs of pigs was studied by electron microscopy during the period 6 hours-14 days after intranasal infection. All bacteria were phagocytosed by polymorphonuclear leucocytes (PMN) and pulmonary macrophages soon after arrival in distal airways and alveoli. Many organisms were destroyed but some survived and later multiplied within phagocytes. Bacteria were also carried in phagocytic cells to lymphatics and pulmonary capillaries, thereby establishing bacteraemia. Between the 5th and 7th days bacteria caused necrosis of the phagocytes and were released into the tissues in very large numbers. Destruction of all types of lung cell was widespread during this period but the Salmonellae did not penetrate pulmonary cells. From the 9th day onwards bacteria in the lung were restricted to circumscribed abscesses and lymphoid tissue developed throughout the lungs. These latter changes coincided with the appearance of circulating specific antibody.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FURNESS G. Interaction between Salmonella typhimurium and phagocytic cells in cell culture. J Infect Dis. 1958 Nov-Dec;103(3):272–277. doi: 10.1093/infdis/103.3.272. [DOI] [PubMed] [Google Scholar]
- Friedberg D., Shilo M. Role of cell wall structure of salmonella in the interaction with phagocytes. Infect Immun. 1970 Sep;2(3):279–285. doi: 10.1128/iai.2.3.279-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOOSLI C. G., BAKER R. F. ACUTE EXPERIMENTAL PNEUMOCOCCAL (TYPE I) PNEUMONIA IN THE MOUSE: THE MIGRATION OF LEUCOCYTES FROM THE PULMONARY CAPILLARIES INTO THE ALVEOLAR SPACES AS REVEALED BY THE ELECTRON MICROSCOPE. Trans Am Clin Climatol Assoc. 1962;74:15–28. [PMC free article] [PubMed] [Google Scholar]
- MERCKX J. J., BROWN A. L., Jr, KARLSON A. G. AN ELECTRON-MICROSCOPIC STUDY OF EXPERIMENTAL INFECTIONS WITH ACID-FAST BACILLI. Am Rev Respir Dis. 1964 Apr;89:485–496. doi: 10.1164/arrd.1964.89.4.485. [DOI] [PubMed] [Google Scholar]
- MORELLO J. A., BAKER E. E. INTERACTION OF SALMONELLA WITH PHAGOCYTES IN VITRO. J Infect Dis. 1965 Apr;115:131–141. doi: 10.1093/infdis/115.2.131. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Halls S. Studies on Escherichia coli enterotoxin. J Pathol Bacteriol. 1967 Apr;93(2):531–543. doi: 10.1002/path.1700930212. [DOI] [PubMed] [Google Scholar]
- Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967 Jan;50(1):109–136. [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Sprinz H. Electron-Microscope Studies of Experimental Salmonella Infection in the Preconditioned Guinea Pig: II. Response of the Intestinal Mucosa to the Invasion by Salmonella typhimurium. Am J Pathol. 1967 Jul;51(1):137–161. [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN G. LABILIZATION AND STABILIZATION OF LYSOSOMES. Fed Proc. 1964 Sep-Oct;23:1038–1044. [PubMed] [Google Scholar]
- Yamamoto I. Ultrastructural changes in mesenteric lymph nodes of mice infected with Salmonella enteritidis. J Infect Dis. 1966 Feb;116(1):8–20. doi: 10.1093/infdis/116.1.8. [DOI] [PubMed] [Google Scholar]