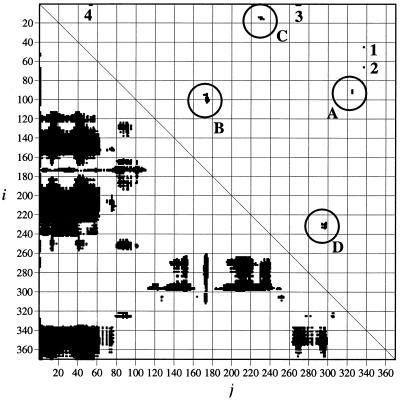
Figure 1.
Dot plot of a double difference distance matrix describing the conformational changes between the open and closed forms of MBP. Below the diagonal line, there is a dot for every pair of Cα atoms that move >4 Å relative to each other. Above the diagonal line, a subset of those pairs, which are within 10 Å of each other in either of the two global conformational states, is shown. This triangle therefore represents the regions of local conformational changes. Groups A–D are spatially separated from the maltose-binding pocket and are predicted to be allosterically linked. Groups 1–4 are either too close to the maltose-binding site (1 and 2) or form part of the partially disordered N terminus (3 and 4).