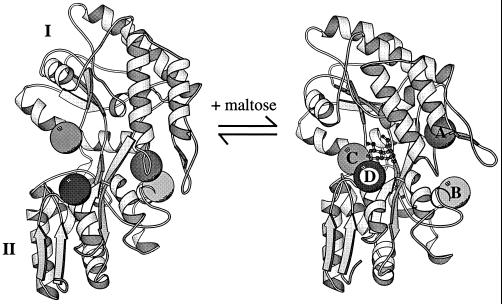
Figure 2.
MBP undergoes a large conformational change upon ligand binding. This involves a relative rearrangement of large rigid subdomains (I and II) best described as a combined hinge-twist movement. The maltose-binding site is formed by the interface between the two subdomains. The shaded spheres (A–D) indicate the regions that are predicted to be allosterically linked to maltose binding.