Abstract
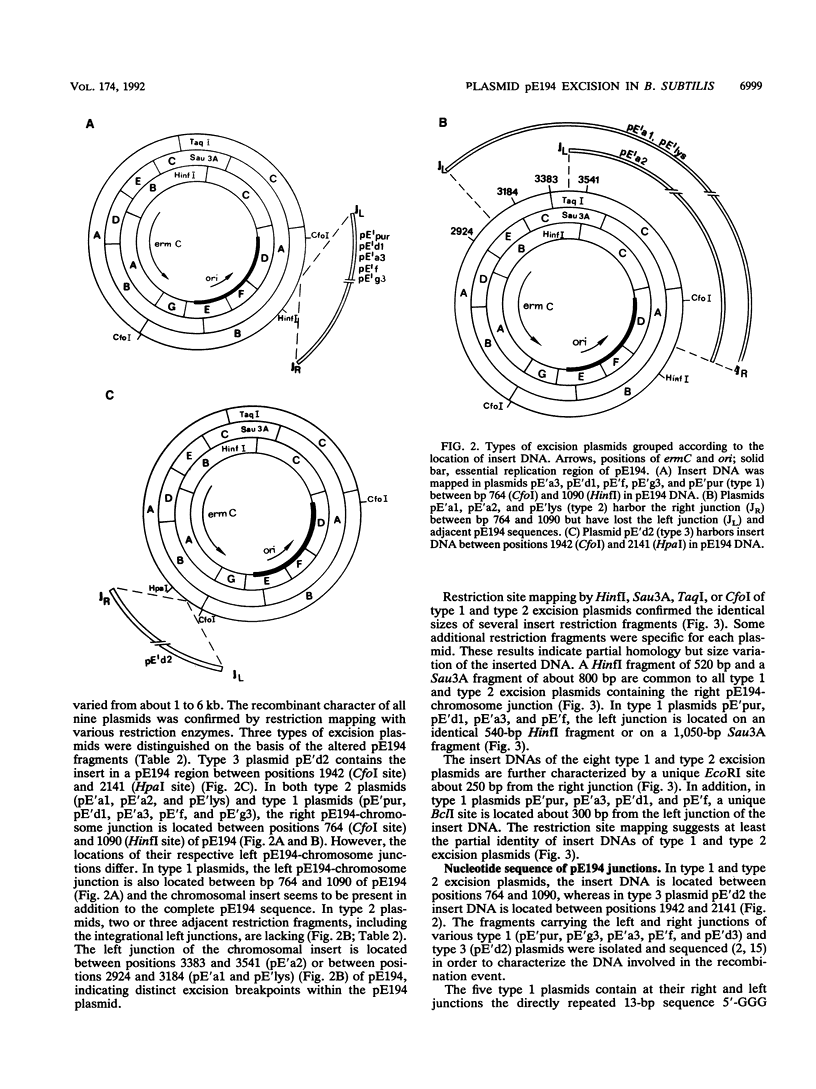
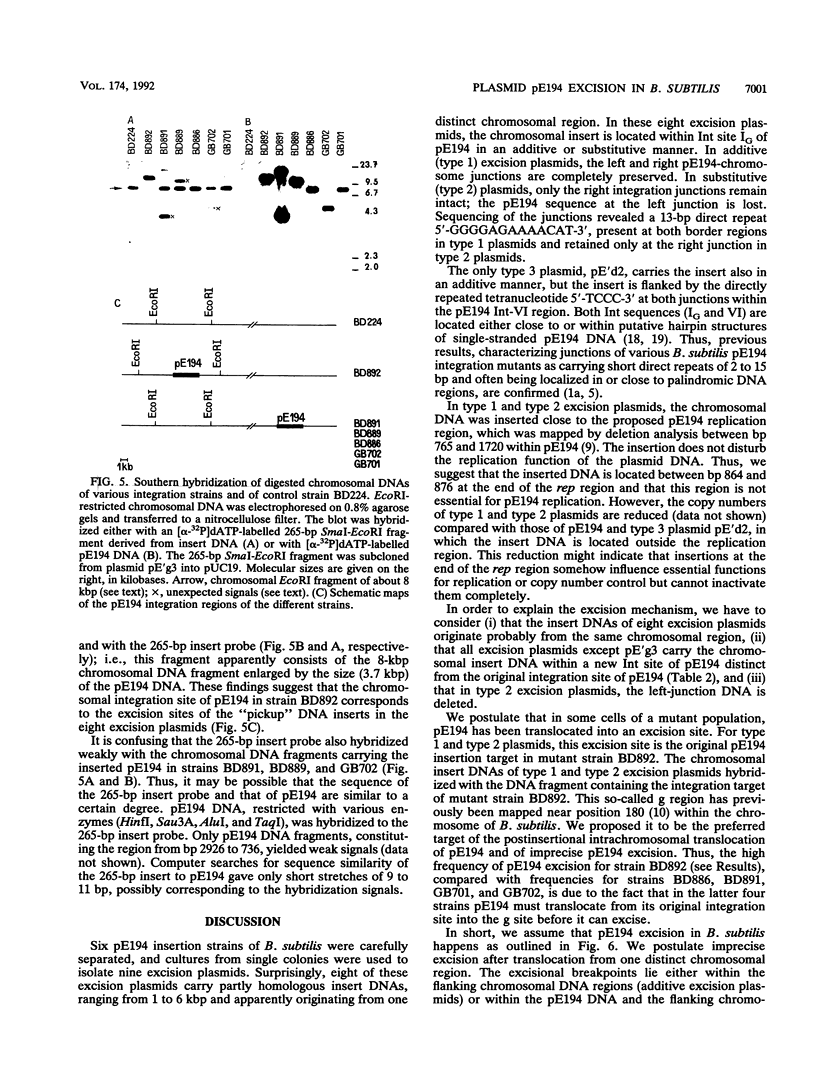
Plasmid pE194 has been shown to be rescued by integration after cultivation of infected Bacillus subtilis recE4 cells at a restrictive high temperature. The plasmid is also spontaneously excised from the chromosome at a low frequency by precise or imprecise excision (J. Hofemeister, M. Israeli-Reches, and D. Dubnau, Mol. Gen. Genet. 189:58-68, 1983). We have investigated nine excision plasmids, carrying insert DNA 1 to 6 kbp in length, either in a complete pE194 or in a partially deleted pE194 copy. Type 1 (additive) excision plasmids have the left- and right-junction DNAs preserved as 13-bp direct repeats (5'-GGGGAGAAAACAT-3') corresponding to the region between positions 864 and 876 in pE194. In type 2 (substitutive) excision plasmids, a conserved 13-bp sequence remains only at the right junction while the left junction has been deleted during the excision process. The type 3 excision plasmid carries at each junction the tetranucleotide 5'-TCCC-3', present in pE194 between positions 1995 and 1998. Although we isolated the excision plasmids from different integration mutants, the insert DNAs of eight independently isolated plasmids showed striking sequence homology, suggesting that they originated from one distinct region of the B. subtilis chromosome. Thus, we postulate that imprecise excision of pE194 occurs most frequently after its translocation from the original insertion site into a preferred excision site within the host chromosome. The imprecise excision from this site occurs at excision breakpoints outside the pE194-chromosome junctions in a chromosomal region which remains to be investigated further.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bashkirov V. I., Khasanov F. K., Prozorov A. A. Illegitimate recombination in Bacillus subtilis: nucleotide sequences at recombinant DNA junctions. Mol Gen Genet. 1987 Dec;210(3):578–580. doi: 10.1007/BF00327215. [DOI] [PubMed] [Google Scholar]
- Chuvpilo S. A., Kravchenko V. V. A simple and rapid method for sequencing DNA. FEBS Lett. 1985 Jan 1;179(1):34–36. doi: 10.1016/0014-5793(85)80185-x. [DOI] [PubMed] [Google Scholar]
- Contente S., Dubnau D. Characterization of plasmid transformation in Bacillus subtilis: kinetic properties and the effect of DNA conformation. Mol Gen Genet. 1979 Jan 2;167(3):251–258. doi: 10.1007/BF00267416. [DOI] [PubMed] [Google Scholar]
- DEMAIN A. L. Minimal media for quantitative studies with Bacillus subtilis. J Bacteriol. 1958 May;75(5):517–522. doi: 10.1128/jb.75.5.517-522.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey L. A., Dubnau D. A. Identification of plasmid and Bacillus subtilis chromosomal recombination sites used for pE194 integration. J Bacteriol. 1989 May;171(5):2856–2865. doi: 10.1128/jb.171.5.2856-2865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol. 1971 Mar 14;56(2):209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Hahn J., Contente S., Dubnau D. Replication and incompatibility properties of plasmid pE194 in Bacillus subtilis. J Bacteriol. 1982 Nov;152(2):722–735. doi: 10.1128/jb.152.2.722-735.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasanov F. K., Bashkirov V. I., Prozorov A. A. Izuchenie integratsii razlichnykh plazmid v khromosomu Bacillus subtilis. Genetika. 1985 Oct;21(10):1618–1626. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Jung R., Hunger H. D. Optimized conditions for solid-phase sequencing: simultaneous chemical cleavage of a series of long DNA fragments immobilized on CCS anion-exchange paper. Gene. 1986;42(1):1–9. doi: 10.1016/0378-1119(86)90144-7. [DOI] [PubMed] [Google Scholar]
- Rüther U. Construction and properties of a new cloning vehicle, allowing direct screening for recombinant plasmids. Mol Gen Genet. 1980;178(2):475–477. doi: 10.1007/BF00270503. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Graham M. Y., Gryczan T., Dubnau D. Plasmid copy number control: isolation and characterization of high-copy-number mutants of plasmid pE194. J Bacteriol. 1979 Jan;137(1):635–643. doi: 10.1128/jb.137.1.635-643.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Riele H., Michel B., Ehrlich S. D. Are single-stranded circles intermediates in plasmid DNA replication? EMBO J. 1986 Mar;5(3):631–637. doi: 10.1002/j.1460-2075.1986.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Riele H., Michel B., Ehrlich S. D. Single-stranded plasmid DNA in Bacillus subtilis and Staphylococcus aureus. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2541–2545. doi: 10.1073/pnas.83.8.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]